Presenters: John Spertus,¹ John Teerlink²
1. Saint Luke’s Hospital, Kansas City, Missouri, USA
2. San Francisco Veterans Affairs Medical Center, San Francisco, California, USA
Disclosure: The EXPLORER-HCM trial was funded by MyoKardia.
Acknowledgements: Medical writing assistance was provided by Amanda Barrell, Brighton, UK.
Support: The publication of this article was funded by MyoKardia, Inc., a wholly owned subsidiary of Bristol Myers Squibb.
Citation: EMJ 2021;6[2]:19-23.
Meeting Summary
Two late-breaking abstracts at the American College of Cardiology’s (ACC) virtual meeting ACC.21 delved further into the data of two pivotal trials, demonstrating the additional benefits of new cardiac treatments for obstructive hypertrophic cardiomyopathy (HCM), heart failure (HF), and heart failure with preserved ejection fraction (HFpEF).
John Spertus, consultant cardiologist and director of cardiovascular education and outcomes research at Saint Luke’s Hospital, Kansas City, Missouri, USA, presented an analysis of health status data collected during the EXPLORER-HCM randomised clinical trial of mavacamten in obstructive HCM.
Consultant cardiologist and director of heart failure and clinical echocardiography at San Francisco Veterans Affairs Medical Center, San Francisco, California, USA, John Teerlink, spoke about a secondary analysis of data from the GALACTIC-HF trial. His team evaluated the modifying effect of baseline ejection fraction (EF) on the treatment effect of omecamtiv mecarbil.
Results from the EXPLORER-HCM Randomised Clinical Trial: Health Status Benefits of Mavacamten in Patients with Symptomatic Obstructive Hypertrophic Cardiomyopathy
John Spertus
In the EXPLORER-HCM trial, mavacamten, a novel direct myosin inhibitor, met its primary endpoints of improving peak oxygen consumption (pVO2),and New York Heart Association (NYHA) classification in obstructive HCM.1 The trial’s secondary endpoints assessed changes in post-exercise left ventricular outflow tract (LVOT) gradient, pVO2, NYHA class, Kansas City Cardiomyopathy Questionnaire (KCCQ), and Hypertrophic Cardiomyopathy Symptom Questionnaire Shortness-of-Breath subscore (HCMSQ-SoB).
Spertus presented the results of a secondary analysis of the results, which sought to better understand the treatment’s impact on health status.
Explaining the background, he said that HCM was a primary myocardial disorder of unexplained left ventricular hypertrophy, often caused by pathogenic variants in sarcomeric genes. Current treatment guidelines focus on improving symptoms and function with β-blockers, verapamil, and disopyramide; more invasive options are reserved for those with refractory symptoms. Mavacamten works by targeting the underlying pathophysiology of HCM and reducing the number of excessive myosin–actin cross-bridges in the sarcomere.
During the EXPLORER-HCM trial,1 251 patients were randomised to either escalating doses of mavacamten (2.5, 5.0, 10.0, or 15.0 mg per day), or to placebo for 30 weeks. They were reassessed 8 weeks after end of treatment (Week 38 end-of-study). Ninety-two per cent of the patients were on HCM monotherapy with β-blockers or calcium channel blockers. Just four people in the treatment group and 16 in the placebo group were not on any background treatment. To provide a more complete understanding of the benefit from the patients’ perspective, Spertus went on, quality of life was assessed prior to randomisation, at the 30-week end-of-treatment point and again at Week 38 end-of-study.
Researchers used the 23-item, disease-specific KCCQ, which explicitly asks patients about their symptoms, physical function, social function, and quality of life. KCCQ overall summary scores (KCCQ-OS) range from zero to 100, with higher scores indicating fewer symptoms, better function, and higher quality of life.
As patients with HCM may not necessarily consider themselves to have HF, Spertus said they took part in cognitive debriefing interviews, during which the study team confirmed the relevance and understandability of KCCQ.
Changes from baseline KCCQ-OS were plotted, using the means and standard errors, over each assessment. The primary analysis focussed on the differences between the treatment and placebo groups at 30 weeks. A responder analysis was also performed to inform the observed mean differences.
Thresholds for responder analysis were defined as:
- ≤-5 points: worsened
- -5 to <5 points: unchanged
- 5 to <10 points: small improvement
- 10 to <20 points: moderate to large improvement
- ≥20 points: large to very large improvement.
Results
“The baseline demographics show great comparability between the treatment and placebo groups, with mean KCCQ-OS scores of 66-plus suggesting a moderately significantly impacted population of patients,” said Spertus.
The primary results demonstrated a “very early separation” between the mavacamten-treated patients and the placebo-treated patients. This was observed as soon as 6 weeks and maintained at 30 weeks. At the end of 30 weeks, there was a 9.1-point greater change in KCCQ-OS among mavacamten-treated patients than placebo-treated patients (Figure 1).
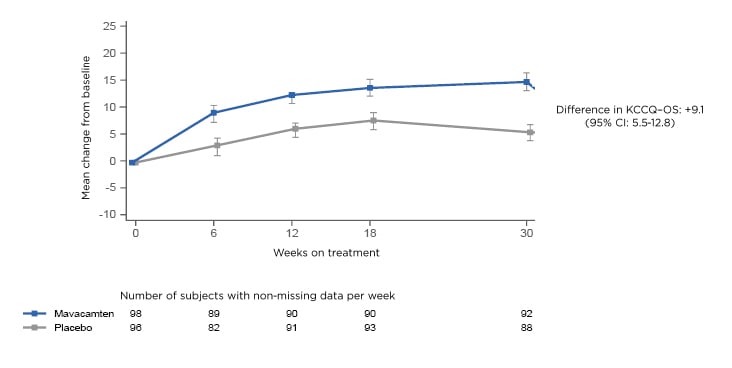
Figure 1: Mean change in KCCQ-OS. CI: confidence interval; KCCQ-OS: Kansas City Cardiomyopathy Questionnaire-Overall Summary Score.
“That was highly statistically and clinically significant,” said Spertus. The team also looked at what happened after the drug was stopped at Week 30. “What we found was when patients came off of treatment, this benefit immediately dissipated. The health status of the mavacamten-treated patients returned to baseline and was no different than the placebo-treated patients,” said Spertus.
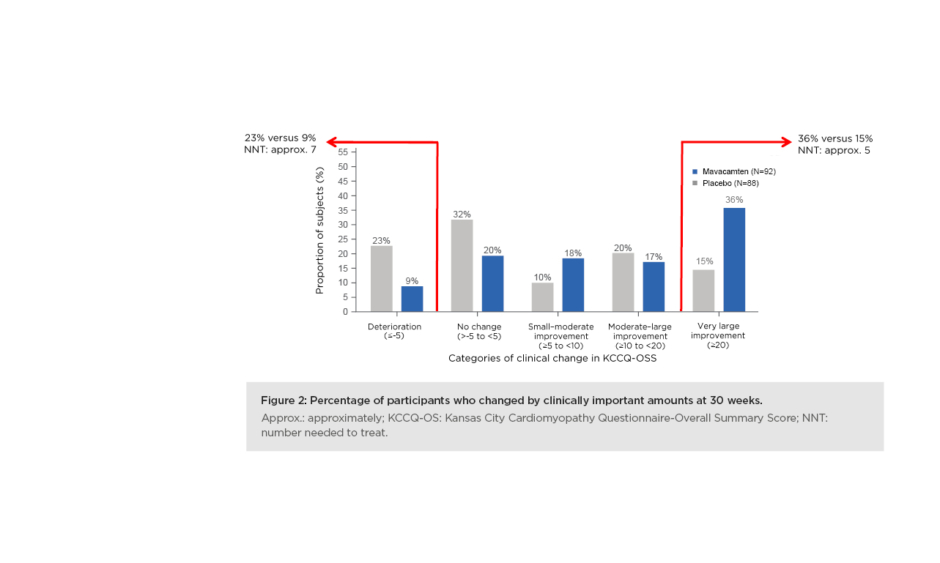
The responder analysis looked at the proportion of patients who got much better and those who got worse. Spertus said: “There was a much larger proportion of placebo patients who got worse over 30 weeks, 23% versus 9% as compared with mavacamten. Most impressively, when you look at the patients who derived a very large improvement from treatment, 36% of the mavacamten-treated patients got substantially better, compared to only 15% of the placebo patients. That 21% absolute difference results in a number-needed-to-treat of only about a five, which is a very large benefit in the health status of patients treated with mavacamten.” (Figure 2).
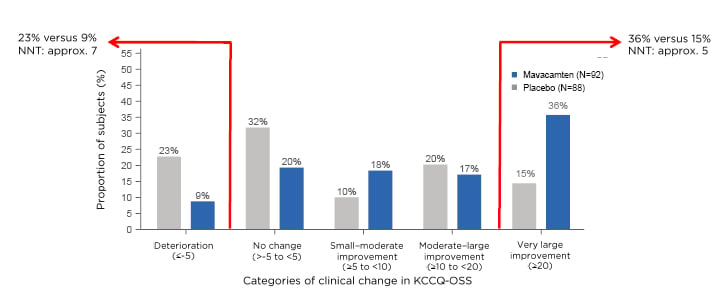
Figure 2: Percentage of participants who changed by clinically important amounts at 30 weeks. Approx.: approximately; KCCQ-OS: Kansas City Cardiomyopathy Questionnaire-Overall Summary Score; NNT: number needed to treat.
Highlighting the limitations of the study, Spertus said this was a short-term trial, so much research is needed before clinicians can be sure of the long-term impact of mavacamten treatment on health status. In addition, there were some missing data; however, the team conducted extensive analyses, and found no observable biases.
Conclusions
Summing up, Spertus said mavacamten was associated with substantial health status improvements in patients with symptomatic obstructive HCM, and that clinicians would only need to treat five people to achieve one improvement of >20 points as measured by KCCQ-OS. “These benefits were observed early after initiating treatment, and they regressed when treatment was stopped.”
Secondary Analysis from GALACTIC-HF: Impact of Ejection Fraction on the Therapeutic Effect of Omecamtiv Mecarbil in Patients with Heart Failure and Reduced Ejection Fraction
John Teerlink
The Global Approach to Lowering Adverse Cardiac outcomes Through Improving Contractility in Heart Failure (GALACTIC-HF) trial achieved its primary endpoint of reducing the risk of cardiovascular death in patients with HF and reduced EF or HF with preserved ejection fraction (HFpEF).2
“But given its mechanism of increasing cardiac function, and based on prespecified subgroup analyses, we assessed the modifying effect of the baseline EF on the beneficial treatment effect of omecamtiv mecarbil,” said Teerlink.
GALACTIC-HF
GALACTIC-HF enrolled patients with symptomatic chronic HF with EF of ≤35%. A total of 8,256 patients were randomised to either omecamtiv mecarbil or placebo, in addition to their usual HF therapy.2 Teerlink described it as “one of the broadest ranges of patients enrolled in a HFpEF trial to date.”
“It was a positive trial, significantly reducing the primary composite endpoint of time to first HF event or cardiovascular death by 8%,” said Teerlink. “For the first time, these results confirm the hypothesis that selectively increasing cardiac function with something such as omecamtiv mecarbil could actually improve clinical outcomes in patients with HFpEF.”
“Within the data, those with more severe HF appeared to have a greater benefit,” he went on.
Secondary Analysis
Teerlink and his team carried out a secondary analysis to investigate the modifying effect of EF on the beneficial treatment effect of omecamtiv mecarbil. While all GALACTIC-HF patients had an EF of ≤35% at baseline, >70% had an EF of ≤30%. Those in the highest quartile (Q4) had an EF of ≥33%.
Patients in the lowest quartile (Q1) had an EF of ≤22%. As well as a lower prevalence of multiple cardiovascular conditions, more nonischaemic cardiomyopathy, and lower BMI, these patients also had greater use of angiotensin receptor neprilysin inhibitors (ARNi), digoxin, ivabradine, and device therapy.
“The primary composite endpoint was the time to first HF event or cardiovascular death,” he explained. “Despite excellent baseline HF therapies, 20–50% of the patients in the placebo group had an endpoint event within one year, with the rate markedly increasing with decreasing EF […] Those in the omecamtiv mecarbil group also had an increasing event rate with decreasing EF, ranging from approximately 22% to 32%.”
In the omecamtiv mecarbil group, there was a progressive beneficial reduction in the absolute event rate with decreasing EF. Treatment also decreased the relative risk of the primary endpoint and had a greater relative treatment effect in patients with lower EF.
With respect to other variables and events of interest, Teerlink said there was no significant difference in systolic blood pressure, serum potassium, or creatinine between the two groups. In addition, serious adverse events and adjudicated arrhythmic and ischaemic event rates were also similar.
Conclusions
In conclusion, Teerlink said that the drug appeared safe, and that fewer than 12 patients would need to be treated to prevent one HF event or cardiovascular death. “Thus, omecamtiv mecarbil represents a novel therapy that holds the promise of improving clinical outcomes in patients with severely reduced EF; the very patients who were the most challenging for us to treat,” he said.