Abstract
Haemopericardium has rarely been described in association with the use of a non-vitamin K antagonist oral anticoagulant. Cardiac tamponade is a life-threatening condition that usually requires urgent pericardiocentesis. Here, the authors report a case of haemopericardium with cardiac tamponade during dabigatran therapy for atrial fibrillation in a patient with chronic coronary syndrome, treated effectively with reversal agent idarucizimab before pericardiocentesis. To the authors’ knowledge, this is only the third report of dabigatran-induced haemopericardium.
Key Points
1. Haemopericardium with cardiac tamponade in a patient receiving dabigatran therapy is rare; the authors have discovered only two previous cases of dabigatran-induced haemopericardium in current literature.
2. Urgent percutaneous drainage is the default strategy for resolving a pericardial effusion, but anticoagulation with dabigatran can complicate the safety of urgent surgical procedures.
3. Idarucizimab acts as a reversal agent for dabigatran, and presents a potential advantage for patients due to reducing the potential haemorrhagic risk of dabigatran and permitting urgent surgical procedures.
CASE REPORT
A 71-year-old male with atrial fibrillation (AF) was admitted to the authors’ emergency department with fever, progressive dyspnoea, and chest pain spreading to the back for 2 days. They suffered from Type 2 diabetes, dyslipidaemia, obesity (BMI: 34 kg/m2), and chronic obstructive pulmonary disease, and they were allergic to penicillin. Some years ago, they had an acute coronary event (related to non-ST elevation-acute coronary syndrome), with right coronary vessel sub-occlusion, which was treated with a percutaneous coronary intervention and insertion of a drug-eluting stent, with moderate left ventricular dysfunction (40%). They had a history of paroxysmal AF for some years, leading to treatment with dabigatran (150 mg twice daily). At the time of prescribing dabigatran, their weight was 98 kg and serum creatinine levels were 1.07 mg/dL (creatinine clearance by Cockroft-Gault formula adjusted for weight was 71 mL/min), so a dabigatran dose of 150 mg twice daily was considered appropriate. The patient’s other daily medications were: aspirin (100.00 mg), due to previous coronary stent implantation; bisoprolol (1.25 mg); amiodarone (200.00 mg); potassium canrenoate (100.00 mg); sertraline (50.00 mg); furosemide (250.00 mg); atorvastatin (20.00 mg); repaglinide (1.00 mg); metformin/linagliptin (850.00/2.50 mg twice daily); and insulin glargine (14 IU).
On admission, their blood pressure was 95/65 mmHg, oxygen saturation 88%, heart rate 100 bpm, and they were anuric. Chest X-ray showed cardiomegaly, mild aortic ectasia, and signs of chronic obstructive pulmonary disease. An ECG demonstrated a low QRS voltage and incomplete left bundle branch block. Haematology results illustrated leukocytosis with neutrophilia (white blood cell count: 10.60×109 /L; neutrophil count: 8.85×103 /µL), low haemoglobin count (9.60 g/dL), and a high level of C-reactive protein (9.89 mg/dL). Renal injury was evident from a blood urea nitrogen level of 0.75 g/L and creatinine level of 2.07 mg/dL (creatinine clearance by Cockroft-Gault formula adjusted for weight was 36 mL/min). Serum potassium was 4.7 mmol/L and the level of sodium was 128.0 mmol/L; liver enzyme tests were normal (aspartate transaminase: 17 U/L; alanine aminotransferase: 14 U/L). Activated partial thromboplastin time (aPTT) was prolonged at 65 seconds, prothrombin time was reduced at 36%, and the international normalised ratio (INR) was measured as 2.03. Slightly elevated troponin levels were also detected (high-sensitivity cardiac troponin T: 36 pg/mL).
Right away, an echocardiogram performed in the emergency room showed a massive circumferential pericardial effusion with initial signs of cardiac tamponade (Figure 1). Regardless of the state of acute renal failure, considering the need to rapidly exclude the presence of aortic dissection, a thoracic angio-CT (Figure 2) was urgently performed. This confirmed a circumferential pericardial effusion of more than 5 cm in diastole with haemorrhagic appearance, as well as a mild dilatation of the proximal ascending aorta (40 mm; 1.72 mm/m2) without signs of dissection. Thoracic tumoural masses were not observed. A dialysis session was scheduled to remove the contrast agent used for angio-CT, if necessary.
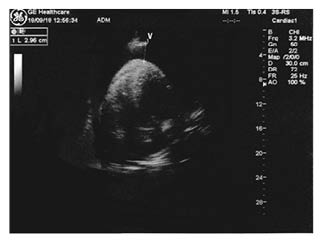
Figure 1: An echocardiogram at the entrance showing haemopericardium on an apical four chamber view.
The patient was transferred to the intensive care unit. The last intake of dabigatran was in the morning, 4 hours before the admission. Idarucizumab (5 g) was administered intravenously; within a few minutes, a subxiphoid pericardiocentesis was performed under local anaesthesia and echo guidance. A 6 F pig-tail catheter (EasyKit®, ab medica, Milan, Italy) was inserted into the pericardium, and 1,400 mL of haemorrhagic fluid was drained. The patient was treated with intravenous fluids and a blood transfusion to increase their blood pressure, after which renal function gradually improved and anaemia slowly resolved. Doses of oral anticoagulant were temporarily stopped, while the antibiotic oral clarithromycin (500 mg once daily) and low doses of the corticosteroid oral prednisone (25 mg once daily) were administered. Fever and inflammation indices reduced (C-reactive protein: 1.50 mg/dL) within a few days, as did renal failure (creatinine: 0.98 mg/dL).
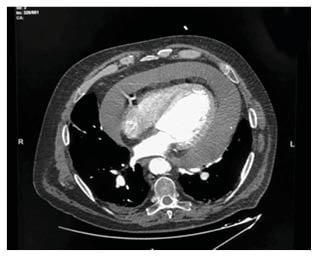
Figure 2: A thoracic angio-CT showing massive pericardial effusion.
Cytology results of the pericardial fluid were negative for malignancy. Fluid microscopy revealed predominantly erythrocytes (740,000 cells/µL) and leukocytes (1,041 cells/µL), with 77% neutrophils, 5.8 g/dL total proteins, 1,492 IU/L lactate dehydrogenase, and a fluid-to-serum lactate dehydrogenase ratio >0.6. All viral serology screenings for Chlamydia pneumoniae, Mycoplasma pneumoniae, and Legionella antibodies; tests for specific neoplasm markers; and an autoimmune disease panel were negative. Subsequently, the echocardiogram showed a resolution of pericardial effusion with residual fibrin stratification (Figure 3).
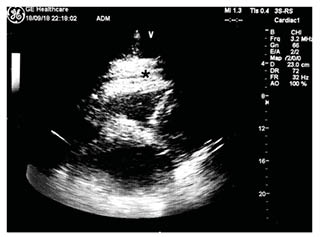
Figure 3: A post-pericardiocentesis echocardiogram showing a resolution of pericardial effusion with residual fibrin stratification at subcostal view.
*Site of residual fibrin stratification.
The patient was discharged 5 days after admission. The therapy prescribed upon discharge consisted of: bisoprolol (1.25 mg), furosemide (100.00 mg), prednisone (12.50 mg), pantoprazole (20.00 mg), clarithromycin (500.00 mg), potassium canrenoate (25.00 mg), atorvastatin (20.00 mg), sertraline (50.00 mg), amiodarone (200.00 mg), dabigatran (110.00 mg twice daily), and antidiabetics, as usual. The ECG on discharge showed sinus rhythm at 70 bpm, a PR interval of 0.20 seconds, an incomplete left bundle branch block, and supraventricular premature beats. At the post-discharge 1-month follow-up, the clinical status of the patient appeared excellent, and no pericardial effusion was found in the transthoracic echocardiogram.
DISCUSSION
Non-vitamin K antagonist oral anticoagulant (NOAC) therapy in patients with AF has been shown to lead to lower rates of stroke and systemic embolism compared with warfarin, as well as variable comparative rates of major bleeding.1,2 At the admission, the patient presented with an altered coagulation status and new-onset renal failure, which might be related to various factors such as a febrile dehydration status with worsening of renal function, cardiac tamponade with obstructive shock, or peripheral hypoperfusion and acute pre-renal insufficiency causing drug accumulation and increased bleeding risk.3 The patient was taking amiodarone, a potent P-glycoprotein inhibitor, and aspirin, which may have increased the risk of bleeding. In renal failure, bleedings are increased two-fold: the accumulation of ureamic toxins during ureamia itself can lead to bleeding episodes.4 Moreover, renal failure contributes to increased bleeding risk because more than 80% of dabigatran etexilate is excreted via the renal pathway.5 The detection of aPTT >90 or INR >2 in blood examination seems to be related to dabigatran overdose in several trials,6,7 as well as in the case outlined here. This hypothetical risk was adequately considered: aspirin was withdrawn at discharge and the dabigatran dosage was reduced to 110 mg twice daily.
The side effects of NOACs are not easy to monitor. There is no laboratory test that can reliably monitor its anticoagulant effect in a similar manner to how the INR is used to monitor dicumarol drugs, or how the aPTT is used for heparin therapy.8,9 An advantage of dabigatran compared with factor Xa inhibitors is the existence of an antidote: idarucizumab, a monoclonal antibody fragment that was approved in 2016 by the U.S. Food and Drug Administration (FDA). Idarucizumab binds to dabigatran with an affinity that is 350 times as high as that observed with thrombin, neutralising its activity within minutes.10 In this case, the administration of idarucizumab permitted a rapid and successful pericardiocentesis after 5 minutes in the setting of haemopericardium, without clinical bleeding or other complications, and not requiring surgical intervention. To the authors’ knowledge, only two cases have previously been reported to date on idarucizumab use in haemopericardium.11,12
The authors’ recommendation for clinical practice is as follows: the use of any anticoagulant is associated with some drug–drug interactions, which may increase the risk of serious bleeding as well as diminish stroke protection.13,14 The European Heart Rhythm Association (EHRA) recommend a structured follow-up of patients on NOACs.15 Echo-guided pericardiocentesis is a feasible procedure for patients in therapy with dabigatran where administration of the antidote idarucizumab occurs immediately before the procedure.16
CONCLUSION
Percutaneous drainage is the default strategy for resolving a pericardial effusion. This case highlights the potential for dabigatran, as well as other NOACs, to cause haemorrhagic effects. However, idarucizumab presents a real advantage for patients in NOAC therapy as it reduces the potential haemorrhagic risk of dabigatran and permits urgent surgical procedures to take place, allowing for better results and fewer complications.







