Abstract
Cardiac surgery procedures for patients following previous pneumonectomy are challenging because of anaesthetic and cardio-surgical technical difficulties. Here, the case of a patient who had received a left-sided pneumectomy 13 years prior as a result of nonsmall cell lung cancer is presented. A mitral edge-to-edge clipping was applied with excellent success in treating severe mitral regurgitation attributable to flail of the posterior mitral valve leaflet (fibroelastic deficiency). Because the heart was severely left-displaced, the use of transoesophageal echo during the preinterventional screening was challenging but feasible, and imaging quality was good. The absence of left pulmonary veins demanded a guide catheter and clip delivery system to be introduced during the procedure through the use of a spiral, preshaped, stiff guidewire. The procedure was performed under general anaesthesia with the patient extubated on a table. No complications arose during the periprocedural period and hospital stay, and after 3 months’ follow-up the patient showed significant functional improvement.
INTRODUCTION
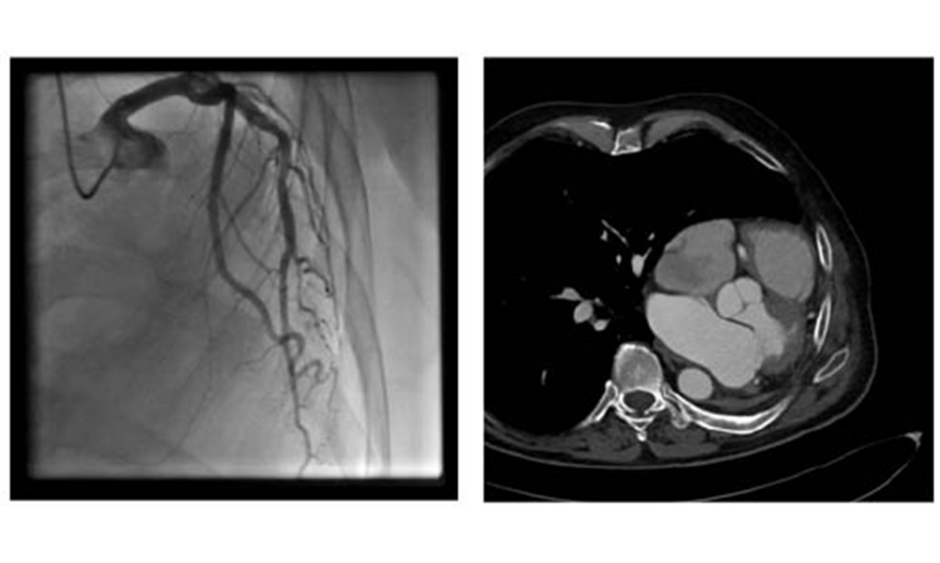
Cardiac procedures and operations following pneumonectomy present with serious challenges, both surgical and anaesthetic. Only a small number of reports of cardiac operations after previous pneumonectomy have been reported.1 General anaesthesia must be performed with special respect to the single lung respiratory status1 whilst also bearing in mind the mediastinal displacement and previous thoracotomy. Common problems concern the respiratory system, where the hyperinflation and unique anatomical situation of the remaining lung presents challenges after pneumonectomy. Anatomically, some changes influence the feasibility of a heart operation and are somehow different after right-sided or left-sided pneumonectomy. After left-sided pneumonectomy, the heart moves laterally to the left and can reach the left thorax wall, as shown in Figures 1A and B. This can result in difficulties with surgical access and operability; therefore, as an alternative to conventional surgical repair in patients with severe mitral regurgitation and indication for repair, edge-to-edge therapies have evolved as a reasonable and superior therapeutic means, especially in patients with functional mitral regurgitation.2 The heart team decision as to whether a conventional or edge-to-edge repair should be attempted is strongly dependent on the perioperative risk and the chance of good operational repair result.3
Figure 1: A) Coronary angiogram showing the left displacement of the heart.6 B) CT showing the left-lateral displacement of the heart after pneumonectomy.
The use of risk stratification scores is routinely used to help decide the better option for patients in need of valvular interventions. According to the latest European guidelines for the management of valvular heart disease, the EuroSCORE II and the Society of Thoracic Surgeons (STS) score4 are the most routinely used, and for this purpose are recommended. Because these scores have some major limitations, decision making is usually an individual process; in this case the usual scores were not able to properly reflect the expected risk by not taking the status after pneumonectomy into consideration in their calculations.
Technical challenges of mitral clipping after left-sided pneumonectomy include the problem of introducing the trans-septal sheath into the left atrium because there is no left lung, and thus no pulmonary veins, to be used to ‘park’ the stiff guidewire. For the surgical phase, difficulties include access problems for minimally invasive right-sided minithoracoto my, in addition to the fact that this is considered a resternotomy. Added to these difficulties are the anaesthetic considerations concerning the single lung status.
REPORT
A 64-year-old male patient (180 cm; 80 kg) was referred to the authors’ centre reporting progressive dyspnea during the previous 6 months, primarily attributable to severe mitral and tricuspid regurgitation (functional New York Heart Association [NYHA] status IV). In accordance to the clinical symptoms and the echocardiographic findings, the neurohormonal activation was present with a NT-proBNP value of 2,759 pg/mL (age adjusted normal range 0.0–57.2 pg/mL). The medical history revealed that the patient had a pneumonectomy followed by adjuvant chemotherapy 13 years ago, these were performed following a diagnosis of nonsmall cell lung cancer. The therapy was considered successful because there was no recurrence of the tumour for >10 years. The patient presented with severe degenerative mitral regurgitation. The monoplane vena contracta was 7 mm, the effective regurgitant orifice area was 0.3 cm2 with a regurgitational volume of 45 mL/beat, and there was flow reflux into the pulmonary veins. Systolic function was slightly reduced (left ventricular ejection fraction: 50%), and there was a lack of leaflet coaptation in the A2/P2 and A1/P1 segments which was attributable to the degenerative process, with a flail leaflet prolapse of the posterior leaflet in the P1-P2 segments.
Coronary angiogram (Figure 1A) results showed no relevant coronary artery disease. Because of their otherwise good general condition, and with the usual risk scores suggesting low operative risk, the patient was primarily referred to the authors’ surgical department for mitral valve reconstruction. However, after chest x-ray there were concerns about the technical operability following a prior pneumonectomy. These included the impossibility of maintaining a minimally invasive approach via a right-sided minithoracotomy, the risk of injury of the compensatory expanded right lung when performing resternotomy, and the risk of postpneumectomy syndrome (Figure 1B).5 Thus, although the patient had a
low STS score of 2.9%, the heart team recommended an edge-to-edge repair using a MitraClipTM (Abbott, Illinois, USA) because of concerns about surgical accessibility. The decision was also triggered by other clinical comorbidities: the status after traumatic splenectomy under anticoagulation with rivaroxaban in the year prior to the intervention, the consecutive development of a postoperative pancreatic fistula, and reports of a slow-growing (10 years) haemangioma in the right lung. These comorbid factors, despite not influencing the STS and EuroScore II scores in many cases, led to the decision in favour of an interventional approach taking into account a nonoptimal reconstructive result.
The procedure was performed using both fluoroscopy and three-dimensional (3D) transoesophageal guidance. The standard right femoral venous approach was used to gain access for the guiding catheter, and a percutaneous closure system (ProGlide [Abbott, Santa Clara, California, USA]) was used afterwards to close the puncture site. No bleeding complications were reported during or after the peri-interventional period.
Specific challenges were encountered throughout the procedure. The first regarded the acquisition of good transoesophageal images attributable to the leftward displacement of the heart, as observed in the chest x-ray and CT. Fortunately, this was easily achieved by performing the transoesophageal echo in the supine position, which was the standard position for the procedure. The authors believe that this might be facilitated by a similar left displacement of the oesophagus to other thorax organs.
A second concern regarded introducing the MitraClipTM guide system safely into the left atrium after trans-septal puncture while avoiding perforation of the left atrium. The routine method to introduce a stiff wire into the left upper pulmonary vein and then place the guide system over this stiff wire was not possible because of the missing pulmonary veins. After several attempts to place the stiff wire (Boston Scientific, Massachusetts, USA) in the left atrium in a safe manner, concerns were raised that the flexible tip would not grant safe introduction of the sheath, and that the stiff-part of the wire would still perforate the wall of the left atrium. This challenge was solved by first introducing a spiral preshaped guidewire (Safari [Boston Scientific, Massachusetts, USA]) into the left atrium and then exchanging the trans-septal sheath for the MitraClip XTR (Abbott, California, USA) guide system and safely advancing it over the preshaped wire into the left atrium. To achieve that used similar technique to that To achieve this, the authors used a similar technique to that used for introducing preshaped wires into the left ventricle during TAVR procedures. First, a pigtail catheter was introduced trans-septally into the left atrium, then the stiff wire was removed and the preshaped spiral guidewire was safely placed into the left atrium: this helped to safely introduce the trans-septal sheath into the area. The first clip was implanted centro-medially, which reduced mitral regurgitation from severe to moderate (Grade 3 to 2; mean pressure gradient after the first clip: 1.68 mm Hg; or mean flow velocity 0.56 m/s). A second clip was then implanted in the centro-lateral position, achieving an excellent result with only minimal (trace) regurgitation. The mitral regurgitation was successfully reduced from severe grade to trivial (3D effective regurgitant orifice area was not eligible because of the small regurgitation amid artefacts from the clips around it). The procedure was performed under general anaesthesia (procedure time was approximately 2 hours; anaesthesia duration was 3 hours and 12 minutes) with the patient extubated on table and no respiratory complications reported (Figures 2A,B, and 3).
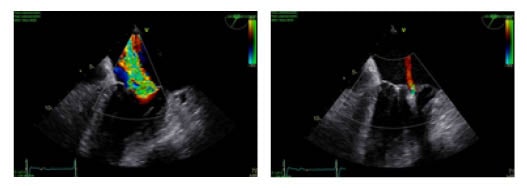
Figure 2: A) Transoesophageal echocardiograph in a high grade mitral regurgitation B) Transoesophageal echocardiograph in a trivial mitral regurgitation following implantation of two clips.
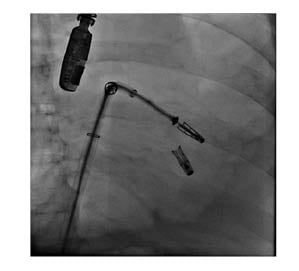
Figure 3: Fluoroscopic view while implanting the second clip.
Postoperatively the patient was transferred to the intensive care unit and stayed for 2 days. No serious complications were reported during the postoperative period. Rate control of the known paroxysmal atrial fibrillation was the cause of the prolonged monitoring; the patient was transferred after a few days to a cardiac rehabilitation unit. Echocardiographic results 3 weeks later showed no residual mitral regurgitation and, interestingly, a reduction in the tricuspid regurgitation from severe to moderate, with a pulmonary artery pressure of 31 mm Hg plus central venous pressure (preoperative value was 45 mm Hg plus central venous pressure). The patient completed the 3-week rehabilitation programme with very good improvement of their functional capacity. The patient was placed in NYHA functional status II 3 months after the intervention.
DISCUSSION
Only few reports exist on cardiac surgery procedures performed after pneumonectomy. In these publications, many concerns are focussed on anaesthesiological and surgical aspects. Kumar et al.1 identified reports of roughly 30 such cardiac operations, most of which involved coronary artery bypass grafting and only few valve surgeries. In this present case, interventional edge-to-edge repair was demonstrated to be a feasible alternative to surgical treatment of severe mitral regurgitation in a patient after pneumectomy.
The use of a spiral curved guide wire enabled a safe introduction of the guiding sheath into the left atrium. Concerns existed regarding echocardiographic imaging in the left displaced mediastinum, but in this case the transoesophageal echo imaging was possible and the images were good. This might be because the oesophagus is also concordantly displaced. The authors still advise this to be checked in the preinterventional screening. By extubating the patient on a table, respiratory complications were avoided, and the early ambulation of the patient prevented further morbidities from the procedure.
SUMMARY
Cardiac surgery procedures for patients after previous pneumectomy are challenging because of anaesthetic and technical difficulties. In this case of a left-sided pneumectomy patient, a mitral edge-to-edge clipping was applied with excellent results in treating severe mitral regurgitation attributable to flail of the posterior mitral valve leaflet (fibroelastic deficiency). Although the heart was severely left displaced, the use of transoesophageal echo was feasible and the imaging results were good. Because of the nonexistent left pulmonary veins, the guide catheter and the clip delivery system was introduced by using a spiral preshaped stiff guidewire. The procedure was performed under general anaesthesia with the patient extubated on table. No complications occurred during the periprocedural period and the hospital stay. After 3 months’ follow-up, the patient showed significant functional improvement.