Coronary chronic total occlusions (CTO), in spite of excellent progress in the field of medicine, remain a great challenge for interventional cardiologists. CTO is defined as the occlusion of a coronary artery with a thrombolysis in myocardial infarction (TIMI) score of 0 flow estimated for >3 months duration. CTO is observed in 15–30% of diagnostic coronary angiograms.1
Due to the low success rates of therapeutic intervention for CTO compared to regular coronary angioplasty, and the greater experience required to perform the associated procedures successfully, chronic occlusions are regarded as a separate type of cardiac lesion. Although revascularisation of CTO is associated with longer procedural times and increased exposure to radiation and contrast, the procedure’s lower success rates can be improved by new CTO strategies. Prof Gerald Werner, CTO expert and former President of the Euro CTO Club, recommends considering chronic occlusions as a target for percutaneous coronary intervention (PCI), similar to any other lesion; however, the technique and experience of the operator must go beyond what is needed for performing PCI in non-occluded coronary arteries.2
In March 2017 at the American College of Cardiology (ACC) Scientific Session, the first randomised trial of stenting versus optimal medical therapy for coronary chronic occlusion was presented.3 DECISION-CTO was a prospective, multicentre, open-label randomised trial comparing the optimal medical therapy with or without stenting for CTO in patients with silent ischaemia, stable angina, or acute coronary syndrome. The primary endpoint of the study was a composite of death, cardiac infarction, stroke, and repeat revascularisation.
During the study, patients were randomised to two groups: 398 to optimal medical therapy and 417 to optimal medical therapy plus PCI of CTO. The success rate of PCI was 90.6%. After 3 years of observation, 19.6% of the optimal medical treatment patients reached the primary endpoint versus 20.6% in the interventional group; this met the noninferiority margin (p=0.008) and the components of the composite endpoint did not differ significantly.3 The second major finding from the trial was that no improvement in quality of life scores or angina occurrence was observed. Moreover, the authors carried out a per-protocol and an as-treated analysis; they observed that event rates did not significantly differ between the two groups.3 DECISION-CTO had four major limitations: lack of clarity regarding how many patients had symptoms or ischaemia after revascularisation of non-CTO lesions; whether myocardial territories due to the CTO were viable at the outset of the study; an almost 20% rate of crossover from the medical therapy arm to the PCI arm; and that the trial was terminated. These limitations significantly decreased the power of the results.
Data from the CREDO-Kyoto AMI registry showed that CTO in a non-infarct-related artery was associated with an increased 5-year mortality in ST-elevation myocardial infarction patients with multivessel disease.4 Similar results were observed in the SCAAR report: CTO was associated with increased mortality, and this correlation was most prominent not only in patients with acute coronary syndrome, but also in patients <60 years of age.5 However, a meta-analysis including 28,486 patients in non-randomised trials confirmed that successful CTO PCI was associated with a lower risk of death, stroke, coronary artery bypass grafting, and less recurrent angina pectoris, when compared with failed procedures.6 Procedural success in the studies included in the meta-analysis was 71% (range: 51–87%). The increasing success rate of revascularisation observed in recent years is noteworthy, with many devices and techniques having been introduced to improve the effectiveness of PCI of CTO.7
Many of the current imaging methods have been developed to overcome the barriers to successful PCI of CTO. Coronary computed tomography angiography is used to predict the success rate of recanalisation and allows better visualisation of the anatomy of arteries, which is not always seen in coronary angiography. Many important predictors of procedure failure have been identified, including severe calcification, bending, blunt stump, the presence of many occlusions, and occlusion length. Coronary computed tomography angiography cannot be recommended for all CTO patients, but this imaging technique should be considered for patients with challenging or ambiguous lesions.
The most basic technique for starting the CTO procedure is via the antegrade approach. In some cases, the use of modern hydrophilic guidewires enables microchannels to be found in the occlusion, allowing access to the true lumen in the distal artery; however, this situation does not occur very often. Typically, other techniques must be used to reopen the artery and, in such cases, occlusion crossing can be achieved by entering the subintimal space, allowing re-entry into the true lumen. Dissection can be completed using a knuckle wire, made by forming a loop in a polymer-covered guidewire (usually of the Fielder variety [ASAHI Intecc Co., Ltd., Aichi, Japan] or the PILOT variety [Abbott Vascular, Santa Clara, California, USA]) and advancing it through the artery.
Re-entry can also be achieved by pushing the wire until it reaches the true lumen. This technique is called subintimal tracking and re-entry (STAR), which is used as the very last resort for crossing the occlusion. It is also possible to carry out a contrast injection into the subintimal space via a microcatheter at the proximal cap to perceive a dissection plane, a process termed contrast-guided STAR. However, access to the distal true lumen is most often achieved by either the mini-STAR technique or limited antegrade subintimal tracking. In the first case, the true lumen is found by advancing the loop of wire distal to the lesion to limit the dissection plane. In the limited antegrade subintimal tracking technique, re-entry is achieved using a stiffer wire (e.g., Confianza [ASAHI Intecc Co., Ltd., Aichi, Japan]).
To ensure better control of crossing the occlusion subintimally, the CROSSBOSS™ (Boston Scientific, Marlborough, Massachussets, USA) catheter was designed. Microdissections created by this fast-rotating catheter allow insertion of a STINGRAY™ (Boston Scientific) balloon and a guidewire into the subintimal space. The STINGRAY guidewire can be pushed towards one of the two side ports of the STINGRAY balloon to reach the true lumen of the artery. These techniques require more procedural and fluoroscopy time, as well as longer stent length, compared to intimal tracking.8 Subintimal techniques can be supported by an intravascular ultrasound, which is useful for finding entry to the true lumen from the subintimal space.
In the case of failure of the aforementioned techniques, other options are available, including the retrograde approach, which was described for the first time in 1990.9 The initial path of the retrograde procedure was via a venous bypass graft, but in 2006 the first paper describing retrograde crossing with the use of septal collateral vessels was published.10 Since then, the retrograde approach has been used worldwide and can be used to treat the most complex CTO lesions. The European guidelines for revascularisation,11 published in 2014, do not consider the retrograde approach as a primary therapeutic option for CTO; however, the Euro CTO Club recommendations1 suggest this technique not only for the second attempt after antegrade failure, but also in cases of very complex CTO with an expected antegrade success rate of <50%.
Due to the fact that the distal cap of the occlusion is softer than the proximal cap, it allows for an easier crossing of the body of the occlusion without losing the true lumen of the artery. Moreover, a distal wire at the end of the occlusion can be used as a marker (marker wire technique), which can aid in the advancement of the antegrade microcatheter (kissing wire technique) and requires less contrast medium. Another successful technique is controlled antegrade and retrograde subintimal tracking (CART). The retrograde-advanced guidewire enters a false lumen, which is then enlarged by inflation of the balloon, allowing the antegrade wire to advance and reach the true lumen. A more commonly used technique is the reverse-CART, a variant of the CART technique in which the antegrade wire reaches the false lumen and the balloon is advanced in an antegrade manner to enlarge the subintimal space where the retrograde wire is positioned. Another retrograde technique is the rendezvous method, which uses two microcatheters moved forward in an antegrade and retrograde manner, allowing the antegrade wire to advance to the distal part of the artery.12 Although very useful, the retrograde approach should be reserved for very experienced operators. This approach can be used after antegrade crossing failure or as the initial approach to CTO therapy, particularly in patients with ambiguous proximal caps, ostial and long occlusions, occlusions with severe proximal tortuosity or calcification, CTO vessels that are difficult to engage, and occlusions with distal major bifurcation.
The successful revascularisation of CTO with PCI in centres with very experienced operators could occur in >90% of cases and complication rates have been shown to be comparable when using the retrograde technique and contemporary antegrade techniques.13,14 The first step in PCI of CTO is careful selection of the collateral vessel because some patients have restricted collateral pathways.15 To find a suitable route via a septal collateral, superselective injection through a microcatheter for better visualisation of the selected vessel is used. Another technique for advancing the wire is called septal surfing. During septal surfing, the operator will attempt to cross collaterals without clear visualisation of the pathway; appropriate guidewire selection during this process is crucial. The dedicated wires for collateral passage are wires from the Sion family (ASAHI Intecc Co., Ltd., Aichi, Japan) and excellent tip control gives even higher chances of a successful crossing. The SUOH3 wire (ASAHI Intecc Co., Ltd.) has a very low tip load (0.3 gram- force), which can be very useful in severely tortuous vessels.
The second step of PCI is the delivery of the microcatheter to the distal CTO and retrograde wiring in the lesion. Development of specialised microcatheters has significantly improved collateral channel crossing and the most commonly used microcatheters for the retrograde approach are Finecross® MG (Terumo, Somerset, New Jersey, USA) and Corsair and Caravel (ASAHI Intecc Co., Ltd.). Microcatheters, thanks to their crossing profile and good trackability, support cross collateral channels, saving procedure time and protecting the collaterals.
Data from 45 centres in Japan showed that predictors for PCI of CTO failures were in-stent occlusion, calcified lesions, and lesion tortuosity. Moreover, lesion calcification was an independent predictor of PCI failure after successful collateral channel crossing.16 In our opinion, supported by the studies discussed, the retrograde technique is effective and safe if performed by experienced operators in centres with a high volume of CTO patients. The long-term outcomes after a successful retrograde procedure have been noted as very good.17 Modern medicine in the field of interventional cardiology offers patients with CTO lesions an interesting spectrum of treatment, and these patients should be given the opportunity to be relieved from ischaemic symptoms to the same extent as patients without CTO lesions. However, our own experiences have shown that non-invasive and invasive cardiologists alike have insufficient knowledge about CTO procedures, which makes them ineligible to properly perform PCI of CTO.18 The available options for CTO treatment are shown in Figure 1.
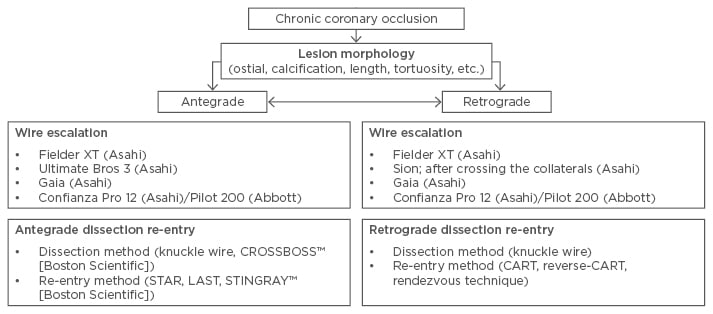
Figure 1: Strategic options for chronic total occlusion treatment.
CART: controlled antegrade and retrograde subintimal tracking; LAST: limited antegrade subintimal tracking; STAR: subintimal tracking and re-entry.
The most challenging aspect for the interventional cardiologist, not a CTO operator, is gaining the proper qualification to perform the procedure and the knowledge of when to refer the patient to a specialised centre. It must be remembered that of all patients with total CTO diagnosed by angiography, only 10% underwent PCI for CTO and 7% were revascularised.19 It is crucial to assemble the entire medical community, not just interventional cardiologists, to disseminate this knowledge about qualification and treatment of coronary CTO to improve the care of coronary patients.








