Abstract
Current clinical guidelines for the diagnosis, treatment, and prevention of heart failure (HF) are the incorporated measure of biomarkers, predominantly natriuretic peptides (NP), cardiac troponins, soluble ST2 (sST2), and galectin-3, all of which serve as surrogate diagnostic and predictive factors. Whether levels of these biomarkers, measured in a longitudinal manner in HF patients, retain their prognostic power over a course of HF therapy and support continuation of these treatments is not fully understood. The aim of this review is to summarise knowledge regarding the use of single and serial measures of cardiac, biological markers as a surrogate endpoint to predict HF-related clinical events. Cardiac biomarkers, predominantly N-terminal segment of brain natriuretic peptide (NT-proBNP) and sST2, are surrogate biomarkers for numerous clinical studies that have assumed a pivotal role in multiple biomarker strategies preceding HF-related outcomes. It has been suggested that biomarker-guided therapy with serial biomarker measures could be a powerful means to appraise composite risk score and predict HF-related outcomes based on therapeutic adjustment. In the future, large controlled clinical trials should be better designed for justification of an individualised strategy for HF therapy.
INTRODUCTION
A contemporary conceptual framework that was proposed to distinguish between different chronic heart failure (HF) patients has highlighted varying responses to therapeutic interventions, especially in patients of different ages and with several comorbidities.1,2 Whether the measure of biomarker serum levels can be determined as a surrogate endpoint for HF-adjusted therapy and suggest clinical outcomes is uncertain, and appears to be controversial in several investigations.3-7 Current HF therapy is associated with improved survival over time in patients with HF-reduced ejection fraction (HFrEF), but not for those with HF preserved (HFpEF) or mid-range (HFmrEF) ejection fraction.3 Conventional approaches for HF treatment aim to affect neuroendocrine modulation using angiotensin receptor-neprilysin inhibitors/angiotensin receptor blockers (ARB), angiotensin-converting enzyme inhibitors (ACEI), beta-blockers, or mineralocorticoid receptor antagonists. Although the high prescription rate of these drugs has helped HF survival in developed countries,4-7 the 5-year survival rate for HF patients in developing countries, regardless of disease phenotype, has failed to exceed 39–43%.8 In fact, maladaptive cardiac remodelling, endothelial dysfunction due to microvascular inflammation, oxidative stress, acceleration of atherosclerosis, and metabolic abnormalities with continuous deterioration of target organ function (heart, vessels, lungs, kidneys, brain, and skeletal muscles) are not tightly controlled by these drugs, and are problems that remain core elements of HF pathogenesis.9,10 In this context, biological markers that reflect the consequent manifestation of HF-evolved pathological abnormalities could be useful in identifying the risk of outcomes.11,12 A similar approach appears to be cost-effective for both inpatients and outpatients with HF.13,14 The aim of this review is to summarise information relating to the use of single and serial measures of cardiac biological markers as a surrogate endpoint to predict HF-related clinical events.
TECHNIQUE FOR INFORMATION SOURCING
Original articles and higher precision reviews written in English and published within the last 5 years were found in MEDLINE (Ovid), EMBASE, PubMed, ScienceDirect, and other databases such as Web of Science, using keywords: “heart failure”, “biomarkers”, “surrogate endpoints”, “natriuretic peptides (NP)”, “soluble suppressor of tumorigenicity-2”, “cardiac troponins”, “galectin-3”. Keywords were designed to be more sensitive using thesaurus tools such as Medical Subject Headings (MeSH) in MEDLINE, and Excerpta Medica Thesaurus (EMTREE) terms in EMBASE. Major descriptors of the articles, titles, and abstract fields were also checked. All selected articles were analysed depending on their quality and relation to the aim of the review and enrolled to the list for further checking depending on whether the references were focussed on target approach.
BIOMARKERS FOR HEART FAILURE
Current clinical recommendations support the use of cardiac biomarkers to stratify, diagnose, and predict clinical outcomes in HF.6,7 Although NP (i.e., BNP: brain natriuretic peptide; NT-proANP: N-terminal pro-atrial natriuretic peptide; NT-proBNP: N-terminal pro-brain natriuretic peptide) are widely used to predict all-cause specific endpoints in HF patients, the 2017 American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Failure Society of America (HFSA) guidelines for the management of HF have incorporated several additional cardiac biomarkers (cardiac troponins, galectin-3, and soluble ST2 [sST2]).7 In fact, the diagnostic and predictive attributes of biomarkers for damage (cardiac troponins) and biomechanical stress (NP, galectin-3, and sST2) in HF have been established. However, the predictive value of growth and differentiation biomarkers, such as growth differentiation factor-15 (GDF-15), in different phenotypes of HF is still under discussion.15,16
Table 1 provides a comparison of conventional and novel biomarkers. Peak concentrations for the majority are applied as risk stratification and diagnostic tools for HF, but NP were predominately recommended for HF guidance and prediction of clinical outcomes. However, the predictive values of NP were sufficiently variable depending on age, comorbidities (including Type 2 diabetes mellitus, abdominal obesity, atrial fibrillation/flutter), and medical treatment.17-19 Novel biomarkers, such as procalcitonin, adrenomedullin/N-terminal fragment of adrenomedullin, microRNA, and other less known biomarkers (human epididymis protein 4, insulin-like growth factor-binding protein 7, soluble CD146, IL-6, endothelial cell derived micro vesicles, and endothelial and mononuclear progenitor cells) require investigation in large clinical trials and to be comparedwith each other, as well as conventional biomarkers.20 Moreover, biomarkers have been distinguished in their ability to predict the onset of different HF phenotypes. Indeed, NP and high-sensitivity troponin strongly predict HFmrEF, whereas NP are better predictors of HFrEF in comparison to HFmrEF and do not differ in their association with incident HFmrEF and HFpEF.21
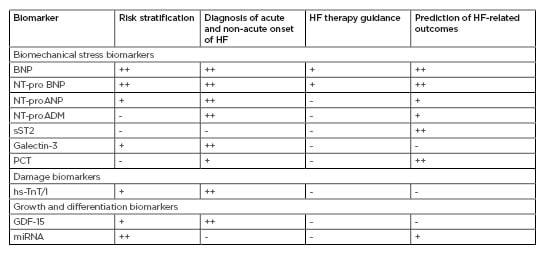
Table 1: Conventional and novel biological markers in heart failure: accompany to clinical hard endpoints.
BNP: brain natriuretic peptide; GDF: growth differentiation factor; HF: heart failure; hs-TnT/I: high sensitivity cardiac troponin T/Index; miRNA: microRNA; NT-proADM: N-terminal fragment of adrenomedullin; NT-proANP: N-terminal fragment of atrial natriuretic peptide; NT-proBNP: N-terminal fragment of brain natriuretic peptide; sST2: soluble suppressor of tumourgenecity-2; PCT: procalcitonin. +: mildly corresponded; ++: moderately corresponded; +++: strongly corresponded
LONGITUDINAL TESTING AND OUTCOMES IN BIOMARKER-GUIDED STUDIES
Whether the levels of these biomarkers, measured in a longitudinal manner in HF patients, retain their predictive power over a course of HF treatment and support HF-guided therapy is not fully understood. Indeed, circulating levels of some biomarkers, such as NP, appear to correlate with body mass, kidney function, and ageing. Another aspect requiring more clarity is the identification of an optimal time window for biomarker measurement. For example, at hospital admission NP levels can better provide predictive value for in-hospital mortality, whereas at discharge, NP levels stronger predict the need for re-admission as opposed to risk of death. Additionally, measurement of NT-proBNP levels after initiation of HF therapy aims to predict a long-term favourable effect of the therapy, but a risk of clinical outcomes is an attributed trend of biomarker changes signifying need for a follow-up. Moreover, fluctuations of serial NP levels received during longitudinal serum biomarker home monitoring in post-acute, decompensated HF patients are extremely high and, as demonstrated in the HOME HF study,22 may never be <30%. In fact, in this study, dispersions of NP data between measures surged depending on the time period after discharge from hospital, with as high as 73.6% being reported at 120 days of ambulatory period. Additionally, some drugs given to HF patients can directly influence metabolism and cardiac biomarkers unrelated to HF severity.22,23 For instance, clinical studies in which HF patients with diabetes were enrolled have revealed that the sodium glucose co-transporter-2 inhibitors, i.e., canagliflozin and empagliflozin, reduce serum levels of NT-proBNP directly through attenuation of kidney clearance and modulation of neprilysin activity, as well as indirectly via lowered fluid retention that improves HF outcomes, including HF-related death, hospital admission due to HF, and all-causes.24,25 Taken together, this evidence shows that serial NP measurements have potential value as an index of emerging clinical deterioration for short-term and long-term periods in HF patients; however, this should be considered on a patient-by-patient basis.26,27 Consequently, there is great interest in other predictive biomarkers that can attenuate the discriminative ability of NP during treatment of HF. However, there are still sufficient differences between various cardiac biomarkers for maintaining HF therapy guidance that require more large clinical trials in the future.
SUGGESTION OF HEART FAILURE-RELATED OUTCOMES WITH CARDIAC BIOMARKERS
There are controversial issues regarding correspondence between dynamics of conventional cardiac biomarkers and clinical outcomes in HF patients. Table 2 summarises data regarding serum levels of cardiac biomarkers and clinical outcomes in HF patients included in large clinical trials.
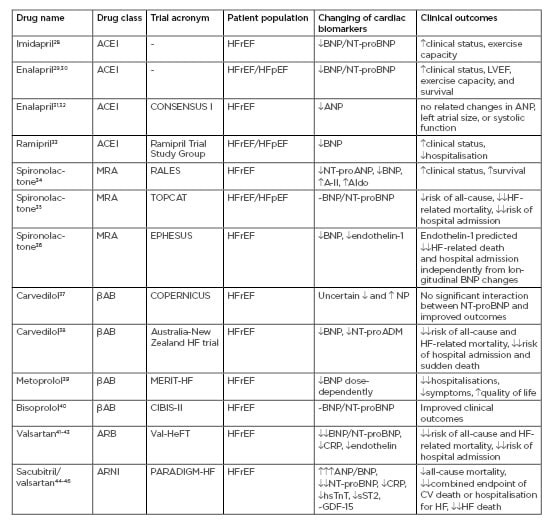
Table 2: Accordance between serum levels of cardiac biomarkers and clinical outcomes in heart failure patients included in large clinical trials.
A-II: angiotensin-II; ACEI: angiotensin-converting enzyme inhibitor; Aldo: aldosterone; ARB: angiotensin receptor blocker; ARNi: angiotensin receptor-neprilysin inhibitor; CRP: C-reactive protein; CV: cardiovascular; GDF: growth differentiation factor; HF: heart failure; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; hsTnT: high sensitivity cardiac troponin T; LVEF: left ventricle ejection fraction;MRA: mineralocorticoid receptor antagonist; NT-proADM: N-terminal fragment of adrenomedullin; NT-proANP: N-terminal fragment of atrial natriuretic peptide; NT-proBNP: N-terminal fragment of brain natriuretic peptide; sST2: soluble suppressor of tumourgenecity-2. : mild increase; : moderate increase;: severe increase; : mild decrease; : moderate decrease;: severe decrease; ~: no effect.
Angiotensin-Converting Enzyme Inhibitors
In the pre-beta-blocker era, clinical trials have demonstrated that ACEI treatment in chronic HFrEF and HFpEF patients decreases plasma BNP and NT-proBNP dose-dependently, and that this effect strongly corresponds with improved clinical status, left ventricular ejection fraction (LVEF), exercise capacity, and survival.28-30 However, the levels of other HF severity biomarkers, such as epinephrine, aldosterone, and endothelin-1, are not significantly affected by ACEI.30 On the contrary, in the randomised CONSENSUS-I controlled trial, ANP levels in an ACEI enalapril cohort had demonstrated a decreasing trend, despite there being no significant correlation between ANP levels and clinical outcomes/LVEF at the end of the study.31,32 The Ramipril Trial Study Group revealed that ramipril was able to reduce the plasma concentration of BNP in HFrEF and improve clinical status.33 Some small clinical studies specifically analysing acute/acutely decompensated HF have demonstrated the clinical benefit of ACEI through declining NP serum levels.47,48 The lack of a strong relationship between longitudinal changes in the levels of NP, survival, and LVEF requires further explanation. It is likely that other biomarkers, including neuropeptides and neurohormones (angiotensin-II, endothelin-1, aldosterone), are not sufficiently modulated by ACEI.34 However, this trial has forced the incorporation of spironolactone into ACEI-based treatment schemes for HF. In this trial, patients included in both spironolactone and placebo cohorts received several ACEI as concomitant therapy, i.e., captopril (63.4% and 62.1%, respectively), enalapril (13.5% and 16.5%, respectively), and lisinopril (15.6% and 13.1%, respectively). It should be noted that during this period, analytical procedures for NP measure had not yet been standardised, and various methods (radioimmunoassay, immunoluminometric, enzymatic, or luminescence immunoassay) with variable analytic accuracies for the determination of other biomarkers were widely used.49 Thus, most clinical studies examining multiple biomarkers, including NP in the same HF patient cohorts using the same highly sensitive analytic methods, have turned out to be necessary. Using this approach, it was established that the decrease in BNP/NT-proBNP among in-patients with acute/actually decompensated HF was associated with a clinically beneficial outcome, whereas no change or an increase in BNP/NT-proBNP levels were related to increased hospital stay and HF-related death.47,50
Mineralocorticoid Receptor Antagonists
The RALES study was the first investigation specifically analysing the mineralocorticoid receptor antagonist spironolactone in patients with severe, congestive HF. In the study, spironolactone exhibited an ability to reduce circulating levels of both BNP and NT-proANP, as well as improving survival in HFrEF patients (LVEF <25%), whereas there was an increase of angiotensin-II and aldosterone I levels, and no change to endothelin-1 levels, in the spironolactone group.34 Later, in the TOPCAT study, spironolactone did not sufficiently reduce the incidence of death from cardiovascular causes, aborted cardiac arrest, or hospital admissions in HFpEF patients, while BNP/NT-proBNP levels were significantly decreased.35
In the EPHESUS trial, HFrEF patients with increased circulating levels of endothelin-1 had exaggerated risk of HF-related death and hospital admission regardless of BNP alterations during treatment with eplerenone.36 It is worth noting that this trial was provided in the HF treatment beta-blocker era, and patients, who had received either spironolactone or placebo, were treated with beta-blockers as a concomitant medication.
Beta-Blockers
The era of beta-blocker implementation in HF therapy began with several clinical trials (e.g., COPERNICUS, IMPACT-HF, MERIT-HF, CIBIS-II, COMET), in which improved survival in HFrEF was completely determined. Unfortunately, the COPERNICUS37 study and an Australia-New Zealand heart failure trial38 have shown that carvedilol effectively decreased all-cause and HF-related mortality rate and hospital admission regardless of BNP/NT-proBNP dynamic. In fact, long-term clinical outcomes in HFrEF patients could be improved even if NP levels were surging. Although pre-treatment plasma BNP/NT-proBNP remained to be independent predictors of all-cause and HF-related mortality and HF-dependent hospitalisation, carvedilol improved survival in HFrEF patients independently from biomarker levels.51 The initiation of metoprolol treatment in HFrEF patients was associated with the soaring of BNP/NT-proBNP levels,52 which was sufficiently pronounced in individuals staged III-IV New York Heart Association (NYHA) functional class.53 Metoprolol did not demonstrate differential impact on survival in HF patients through dose-dependence, while serum levels of BNP were changed dose-dependently.40 In the CIBIS-II trial, bisoprolol did not significantly impact BNP levels, while it did improve survival and attenuate the clinical status of HFrEF patients.40 The results of the STARS-BNP Multicentre Study have shown that the combined use of ACEI and beta-blockers can reduce BNP levels in HFrEF patients, but this effect appeared to be dose-dependent and mean dosages of both drugs were significantly higher in the patients with best BNP control; this contrasts with the mean increase in loop diuretic furosemide dosage that was similar in both groups (best and worst BNP control, respectively).54 In fact, the best BNP control was reported in patients with the lowest risk of HF-related death and hospital stay for HF.55 Overall, patients with severe HFrEF and levels of BNP >1,000 pg/mL had a 40% risk of acute decompensation of HF after an initiation or increase of beta-blocker therapy.55 Thus, uncertain evidence of contra directed BNP/NT-proBNP levels in severe HFrEF, and data that confirmed decreasing serum levels of these biomarkers in mild-to-moderate HFrEF patients, reflected a risk of potential beta-blocker side effects as opposed to a negative impact of the drugs on HF evolution in long-term perspectives.
Angiotensin-Receptor Blockers
ARB appeared to be promising drugs for improved survival in HF, offering benefits over ACEI. The first results of ARB implementation have shown positive effects on decreeing serum levels of biomechanical stress markers such as NP. In the Val-HeFT study, serial measures of BNP/NT-proBNP concentrations taken at 4-month intervals in HFrEF patients treated with ARB valsartan were performed.41 The results of the study illustrated a superior strategy for the risk stratification of stable patients with chronic HF using the determination of NT-proBNP serum levels. However, there was a small proportion of the HF patients showing increased BNP/NT-proBNP serum levelsdespite contemporary treatment, including ARB valsartan, beta-blockers, spironolactone/eplerenone, loop diuretic, and non-frequently digoxin.42,43 Investigators concluded that the trend in soaring NP levels was an independent predictor of all-cause and HF-related mortality and hospitalisation, regardless of the kind of HF therapy.43 Interestingly, non-responders for ARB therapy in the Val-HeFT study that associated with increased levels of BNP/NT-proBNP had frequently higher levels of endothelin-1 and CRP than those who had exhibited low levels of NP.42,56 Additionally, serial measures of NT-proBNP were done in 3,480 HFpEF patients in the I-PRESERVE study to identify whether ARB-based treatments for HF have a distinguishing impact on morbidity and mortality across serum NP levels.44 The investigators have ascertained that the beneficial effect of ARB irbesartan on clinical outcomes, including all-cause mortality, HF-related death, sudden death, or HF hospitalisation was found alongside increased NT-proBNP levels at baseline.
Angiotensin Receptor-Neprilysin Inhibitors
A new class of angiotensin receptor-neprilysin inhibitor agent called sacubitril/valsartan in addition to a conventional treatment in the PARADIGM-HF trial led to a 4-fold increase in ANP/BNP due to mediating metabolism of neprilysin and moderate decreases in NT-proBNP.45 Therefore, cardiac troponin, hs-CRP, and sST2 levels, which were unrelated to neprilysin activity, also decreased. These changes accompanied fewer all-cause and HF-related deaths or HF admissions in patients with NT-proBNP levels lowered to 1,000 pg/mL compared to patients with levels that remained above this value.46 Another result of the PARADIGM-HF trial was an effect on GDF-15 dynamics. Although the levels of GDF-15 at baseline were associated with higher mortality risk, the combined endpoints of CV death and hospitalisation for HF and HF death, and changes in GDF-15, were not influenced by sacubitril/valsartan.57 Yet, longitudinal changes in galectin-3 were not associated with the natural evolution of HF.58 Consequently, it is not fully clear whether this biomarker could be useful for guided therapy in HF.
Biomarker-Guided Therapy as a Component of Individual Approach in Heart Failure
Early clinical trials regarding NP-guided therapy for HF have reported inconsistent results, frequently dependent on statistical power, limiting sample sizes, and repeating measures of biomarkers.59,60 Therefore, HF patient cohorts involved in the studies have displayed large biological heterogeneity at the levels of CV risk, metabolic profile, and adverse events. The GUIDE-IT study indicated that the goal of HF treatment could be achieving a target NT-proBNP level of <1,000 pg/mL.61 The strategy of HF therapy, which is based on serial NT-proBNP measures, did not improve HF clinical outcomes.62,63 In contrast, the Bio-SHiFT study64 has revealed that individual patterns of longitudinal changes in multiple biomarkers, including NT-proBNP and hs-CRP, may be useful for a prognostication of HF-related outcomes and response to treatment. Additionally, the results of the TIME-CHF trial have shown that the beneficial effect of NT-proBNP-guided treatment was found only in HFrEF patients aged <75 years, and not in those aged ≥75 years.64 This positive impact of NT-proBNP-guided therapy was associated with reduced risk of recurrent HF-related and all-cause hospital admissions and all-cause death.65 Thus, NP revealed a potential association with HF clinical outcomes predominantly in HFrEF patients. Attempts to improve predictive values of NP mostly affect implementation of multiple biomarker models shaping from NP, hs-CRP, sST2, galectin-3, hs-troponin T, and several novel biomarkers (miRNA, GDF-15, metabolic factors).66 Lastly, although meta-analyses that depicted NT-proBNP-guided HF treatment had produced controversial results,67,68 the combination of NP and sST2 appeared to be reinforced in a new investigation to clearly explain the meaningful longitudinal changes of markers in the treatment programme of HF patients aimed at improving prognosis. In fact, future clinical trials are needed to provide a direct comparison between traditional and novel biomarkers in preceding HF clinical outcomes and adjusting treatment programme to improve prognosis. It is likely that the combined biomarker measure could significantly improve prediction of clinical outcomes in HF patients as opposed to single biomarker measures, and open new perspectives for biomarker-guided targeted HF therapies.
CONCLUSION
Cardiac biomarkers, predominantly NT-proBNP and sST2, are surrogate biomarkers for numerous clinical studies that have assumed a pivotal role in multiple biomarker strategies for preceding HF-related outcomes. It has been suggested that biomarker-guided therapy with serial biomarker measures could be a powerful tool to appraise composite risk score and predict HF-related outcomes based on therapeutic adjustment. In the future, large controlled clinical trials should be better designed for a justification of individual strategy in HF therapy.








