Abstract
Background: Pre-eclampsia is a common condition that causes significant morbidity and mortality in pregnant women; the occurrence of cardiovascular complications aggravates the disease. Efforts have been made to predict the complications of pre-eclampsia, but some modalities, such as echocardiography and biomarkers, are neither available nor widely feasible for use by healthcare providers, especially in developing countries. On the other hand, ECG is cheap, noninvasive, widely available, and already routinely performed for pre-eclampsia. The role of ECG in predicting cardiovascular complications in pre-eclampsia patients is not known.
Objective: This study aimed to investigate the role of ECG in pre-eclampsia diagnostics and simple clinical parameters in pre-eclampsia patients with and without cardiovascular complications.
Methods: This cross-sectional, analytical study used retrospective data from medical records of patients with pre-eclampsia from the Dr Kariadi General Hospital, Semarang, Indonesia, from January 2016–July 2017. Bivariate association between demographic, clinical, laboratory, and ECG results with the occurrence of cardiovascular complications was tested; this continued with logistic regression.
Results: Sixty-eight pre-eclampsia patients were identified, with a mean age of 30.2 years. Cardiovascular complications occurred in 16 patients (23.5%), with 14 patients exhibiting pulmonary oedema. In univariate analysis, haemoglobin level and heart rate showed a significant association with the occurrence of cardiovascular complications (p=0.035 and 0.033, respectively). No significant independent predictor was found in multivariate analysis.
Conclusion: This study showed that ECG parameters were not able to predict cardiovascular complications in pre-eclampsia patients. Nevertheless, there was a significant association between heart rate and haemoglobin level with cardiovascular complications in pre-eclampsia.
INTRODUCTION
High maternal mortality rate (MMR) is a health problem in many developing countries, and high MMR reflects the quality of healthcare during pregnancy and puerperium.1 The MMR in Indonesia, with rates as high as 228 per 100,000 live births, is one of the highest in South East Asia, and pre-eclampsia is the major contributor to maternal and perinatal morbidity and mortality, with an incidence of 128,273 per year (5.3% of all pregnancies).2,3 Furthermore, 99% of maternal deaths occur in low and middle-income countries.4 This is associated with the occurrence of pre-eclampsia-related complications, including cardiovascular complications,5 with a delay in diagnosis and treatment resulting in a higher rate of death.6 Cardiovascular complications, such as pulmonary oedema, occur in 4.3%–15.0%7-9 of pre-eclampsia patients and are the cause of 17.2% maternal deaths.10 Women with pre-eclampsia or eclampsia experienced a 42% increased risk of cardiovascular disease, and those who developed cardiovascular disease experienced a 5-fold increase in healthcare-related costs during follow-up.11
These findings led to the development of a predictive model of pre-eclampsia’s complications.12-16 However, some examination techniques used in the prediction models, such as echocardiography and biomarkers, are not universally available, and are not feasible for use in developing countries. On the other hand, ECG has been used routinely in pre-eclampsia to identify cardiovascular complications that have already occurred, yet the role of ECG in predicting those occurrences is not yet clear. This study aimed to investigate the association between ECG and clinical parameters with the occurrence of cardiovascular complications in pre-eclampsia patients.
METHODS
This analytical cross-sectional study used retrospective samples from the medical records of patients who were diagnosed with pre-eclampsia at the Dr Kariadi General Hospital, Semarang, Indonesia, from January 2016–July 2017. Diagnosis of pre-eclampsia as the inclusion criteria was based on current guidelines,17 and samples with cardiovascular complications upon admission, chronic hypertension (defined as blood pressure [BP] >140/90 mmHg before pregnancy or before 20 weeks’ gestation), or heart disease (congenital, valvular, ischaemic, and/or arrhythmia) were excluded from the study. The study was approved by the Education and Research Board of Dr Kariadi Hospital.
The independent variables were demographic, clinical, laboratory, and ECG parameters, with the occurrence of cardiovascular complications manifesting as pulmonary oedema, heart failure, or myocardial ischaemia during the peripartum period as the dependent variables.18
The demographic data used were age and education level; the clinical data used were gestational age at diagnosis, weight, height, antenatal care frequency, systolic and diastolic BP, and nulliparous or not. Later, BMI and mean arterial pressure ([systolic BP+(2 x diastolic BP)]/3) were calculated. The laboratory data analysed were haemoglobin, ureum, creatinine, and lactate dehydrogenase.
Electrocardiography
The standard 12-lead ECG performed during admission was analysed both qualitatively and quantitatively. Heart rate (HR) was calculated by dividing 1,500 by the R wave-to-R wave small square interval. The electrical axis was determined using the method presented by Baltazar et al.19 PR interval was measured as the time between the beginning of the P wave and the beginning of the QRS complex in milliseconds. QRS duration was measured from the start until the end of the QRS complex in milliseconds. Sokolow–Lyon voltage was taken from the bigger summation between S wave in V1 and R wave in V5 or V6.20 QT interval was measured as the time from the start of the Q wave to the end of the T wave and corrected by HR using Bazett’s formula.21
The criteria for left atrial abnormality were 1) widely notched P wave (40 ms); 2) terminal negative component of the P wave in lead V1 with duration ≥40 ms and amplitude ≥-0.1 mV; or 3) P wave duration ≥120 ms.22
Myocardial ischaemia was determined by either ST elevation at the J point in two contiguous leads, with the cut-points ≥0.1 mV in all leads other than leads V2–V3 where different cut points applied: ≥0.2 mV in men ≥40 years, ≥0.25 mV in men <40 years, or ≥0.15 mV in women; or horizontal or down-sloping ST depression ≥0.05 mV in two contiguous leads and/or T inversion ≥0.1 mV in two contiguous leads with a prominent R wave or R:S ratio >1.23
Data Analysis
Statistical analysis was performed using SPSS version 23 (IBM SPSS Statistics, Armonk, New York, USA). Data are reported as a percentage or mean ± standard deviation according to the type of the data. Comparative tests between groups were performed using independent T-test and Chi-square tests, with Yates correction if the independent variable was numeric and nominal, respectively. If the data were not distributed normally and normalisation failed, Mann–Whitney and Fisher tests were used in place of the T-test and Chi-square tests, respectively; association would be considered significant if p<0.05. If the univariate analysis yielded more than one association with p<0.25, multivariate analysis was performed to determine the independent predictor(s).
RESULTS
During the study duration, 115 samples were obtained, and 47 of them were excluded; 30 were excluded due to chronic hypertension, 3 due to congenital heart disease (all of which were atrial septal defect), 9 due to valvular heart disease (3 rheumatic mitral stenosis, 6 mitral regurgitation), and 5 due to pre-existing arrhythmia (4 atrial fibrillation, 1 junctional bradycardia). Final analysis included 68 pregnant women with pre-eclampsia.
Characteristics and Outcome
Characteristics of the patient population are shown in Table 1. Cardiovascular complications occurred in 16 patients (23.5%). More specifically, heart failure with pulmonary oedema occurred in 14 patients, and heart failure without pulmonary oedema and heart failure with myocardial ischaemia occurred in 1 patient each, respectively.
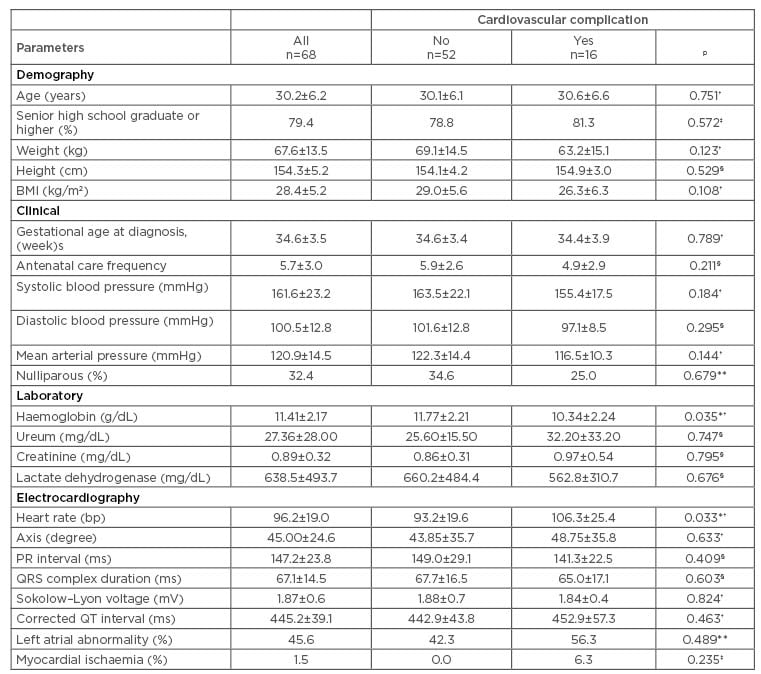
Table 1: Patients’ characteristics and association between groups with and without cardiovascular complication.
Values are shown as mean±standard deviation or percentage.
*p<0.05
†Independent T-test for same variance
‡Fisher test
§Mann–Whitney test
**Chi-square test with Yates correction.
Comparative Analysis
The results of the comparative analysis are reported in Table 1. Only haemoglobin (p=0.035) and HR (p=0.033) had a significant association with the occurrence of cardiovascular complication; nevertheless, in those with cardiovascular complications, left atrial abnormality and myocardial ischaemia were more prevalent and the corrected QT interval tended to be longer.
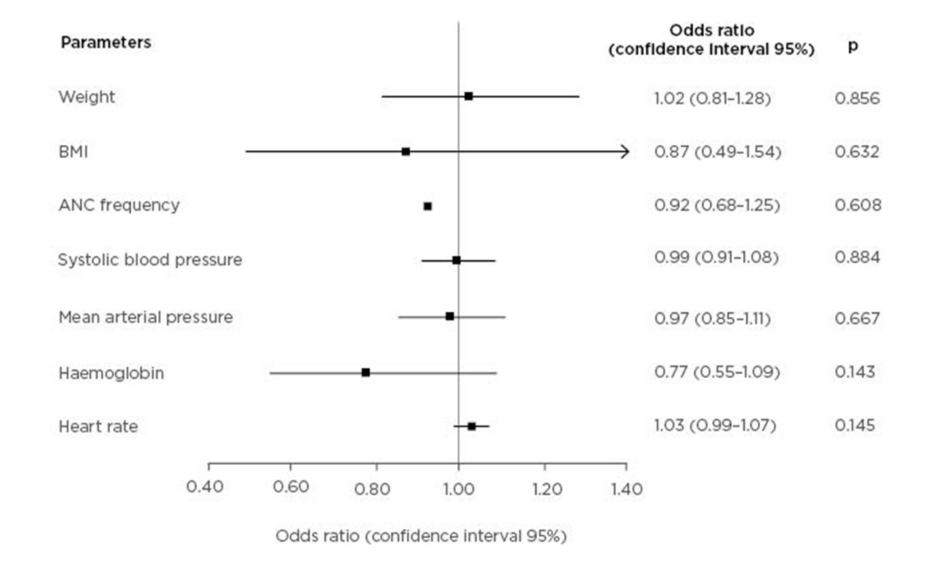
A multivariate analysis on weight, BMI, antenatal care frequency, systolic BP, mean arterial pressure, haemoglobin, and HR showed that no variable is a significant independent predictor of cardiovascular complications, as shown in Figure 1.
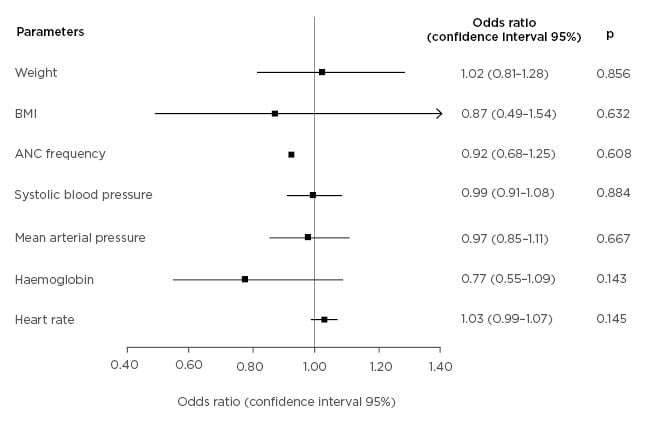
Figure 1: Forest plot of cardiovascular complication predictors in pre-eclampsia.
ANC: antenatal care.
DISCUSSION
The occurrence of cardiovascular complications contributes to morbidity and mortality in pre-eclampsia patients. With prediction of the condition in mind, this study found that HR and haemoglobin levels were significantly associated with the occurrence of cardiovascular complications.
In this study, cardiovascular complications occurred in 23.5% of patients, a greater proportion than is reported in any of the published literature. The incidence of pulmonary oedema as the cardiovascular complication in the pre-eclampsia population varies, from 0.5% in all pregnancies24 to 4.3–15.0% in patients with severe pre-eclampsia or HELLP syndrome,5,8,9,25 and 5.0–33.0%26,27 in patients with eclampsia. This discrepancy might be caused by the location of the study; the Dr Kariadi General Hospital is a national referral hospital and, thus, the patient population tends to suffer from more severe disease, while patients with less severe and non-complicated disease would be already treated in lower-tier hospitals.
Pulmonary oedema is the most common cardiovascular complication of pre-eclampsia in this study, in accordance with previous studies.25,28 The physiological changes in the maternal cardiovascular system, including increased plasma blood volume, cardiac output, HR, and capillary permeability, and a decrease in plasma colloid osmotic pressure, are exaggerated in pre-eclampsia and so predispose patients to developing pulmonary oedema. Plasma colloid osmotic pressure decreases after delivery, which may be caused by excessive blood loss and fluid shifts secondary to increased capillary permeability. These changes may explain why most occurrences of pulmonary oedema in pre-eclampsia develop after delivery.25,29,30
von Dadelszen et al.12 developed the fullPIERS model, which aimed to identify risk factors for complications in pre-eclampsia patients, but HR and haemoglobin were not included in the study.
Similarly, Thornton et al.15 studied the incidence and causative factors of acute pulmonary oedema in patients with hypertensive disorders of pregnancy, and HR and haemoglobin levels were not considered in this study.
Angeli et al.31 reported that HR was not able to predict the risk of hypertensive disorders during pregnancy; furthermore, Raffaelli et al.32 also showed no association between HR and the cardiac effects of pre-eclampsia. It is possible that the increase in HR was not the cause or risk factor but a manifestation of cardiovascular complications.33 This bias was minimised by using the initial ECG and excluding patients presenting with cardiovascular complications upon admission. The increase in HR is a compensatory mechanism to preserve cardiac output in this population and reflects the severity of pre-eclampsia; studies showed the development of left ventricular dysfunction,34,35 increased aortic stiffness,36 and decreased stroke volume.37 As previously discussed, the incidence of cardiovascular complications is directly proportional to pre-eclampsia severity,7-9 and this relation forms the basis of association between HR and cardiovascular complications.
Despite the limited research into ECG changes during pregnancy, there is evidence that pregnancy affects ECG and those changes are reversible at the end of or after pregnancy.38 A similar scarcity of data also applies to women with hypertensive disease of pregnancy. Present evidence has demonstrated that hypertensive disorders of pregnancy was associated with changes of the P wave morphology and QT interval. Angeli et al.31 reported that left atrial abnormality diagnosed by P wave changes in lead V1 is an independent predictor of hypertensive disorder during pregnancy. On the other hand, Raffaelli et al.32 reported that prolonged corrected QT interval and QT dispersion in pre-eclampsia patients increased the risk of cardiovascular events. However, this study did not yield a significant value of standard ECG parameters for predicting the risk of cardiovascular complications. This might be caused by the inherent limitations of ECG. The left atrial abnormality criteria used has a sensitivity of 4–52% and specificity of 53–100%,22 meanwhile Sokolow–Lyon voltage criteria for left ventricular hypertrophy has a sensitivity of 18–22% and specificity of 79–92%.39 The Bazzett formula for correcting QT interval tends to overestimate the corrected result if the rate is high.40 These inherent weakness, combined with the small sample size, might influence the ability of the study to achieve statistical significance. Nevertheless, the corrected QT interval was longer and left atrial abnormality and myocardial ischaemia was more prevalent in those who progressed to cardiovascular complication. HR, the only ECG parameter with significant univariate association, can be easily replaced with standard physical examination, hence further weakening the role of ECG in this context.
Haemoglobin level, the other significant variable in univariate analysis besides HR, is related to pre-eclampsia and/or eclampsia,41-43 but there is no published literature currently available regarding the role of haemoglobin levels in cardiovascular complications. In their observational study, Patra et al.43 reported on the incidence of heart failure in pregnant women with severe anaemia (haemoglobin <5 g/dL) to be as high as 18%. Low haemoglobin levels during pregnancy predisposes the patient to infection,44 hypoxia,45 oxidative stress,46 endothelial dysfunction, and inflammatory response.47 Furthermore, a decreased haemoglobin level accompanied by haemoconcentration can cause vasoconstriction and endothelial dysfunction, which later increased the vascular permeability.41 Decreased haemoglobin and haemoconcentration were associated with the pathogenesis of pulmonary oedema,33 hence the association between haemoglobin and cardiovascular complication.
LIMITATIONS AND FUTURE DIRECTIONS
This research was limited by a small sample size, the use of secondary retrospective data, not using a combination of ECG criteria to improve sensitivity and specificity, and manual interpretation of ECG, which allows for inter and intra-observer variability. Further research with a larger sample size, prospective research design, and use of more comprehensive parameters is needed. Further studies may allow for the development of a cheaper, non-invasive, and more widely available tool that can be used to predict cardiovascular complications in pre-eclampsia.
CONCLUSIONS
Even though HR and haemoglobin levels showed significant association with cardiovascular complication in univariate analysis, this study did not find ECG useful in predicting cardiovascular complication. The use of parameters, such as increased HR, prolonged corrected QT interval, and left atrial abnormality, for predictive purposes still requires further study.