Abstract
Heart failure is a chronic, progressive, and incurable disease. Cardiac cachexia is a strong predictor of poor prognosis, regardless of other important variables. This review intends to gather evidence to enable recognition of cardiac cachexia, identification of early stages of muscle waste and sarcopenia, and improve identification of patients with terminal heart failure in need of palliative care, whose symptoms are no longer controlled by usual medical measures. The pathophysiology is complex and multifactorial. There are many treatment options to prevent or revert muscle waste and sarcopenia; although, these strategies are less effective in advanced stages of cardiac cachexia. In these final stages, symptomatic palliation plays an important role, focussing on the patient’s comfort and avoiding the ‘acute model’ treatment of aggressive, disproportionate, and inefficient care. In order to provide adequate care and attempt to prevent this syndrome, thus reducing its impact on healthcare, there should be improved communication between general practitioners, internal medicine physicians, cardiologists, and palliative care specialists since heart failure has an unforeseeable course and is associated with an increasing number of deaths and different levels of suffering.
INTRODUCTION
Heart failure (HF) is a progressive organ failure disorder, characterised by dyspnoea, fatigue, depression, and fluid retention, and affects ≤2% of the Western population.1,2 It is a dynamic situation that, in the later stages, has high mortality rates. It is associated with several hospital readmissions due to its chronic and progressive disease evolution.3-6 There is a gradual loss of functional capacity and self-sufficiency of the patient, which is portrayed by a pattern of sudden worsening without complete recovery (Figure 1).7-8 In general, elderly patients with HF have other comorbidities, which cause different outcomes for these patients.9
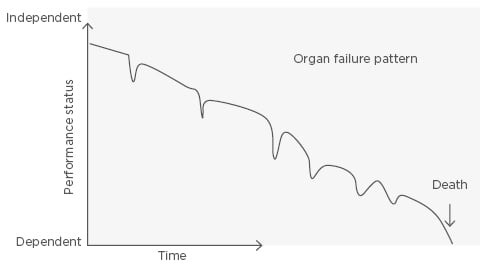
Figure 1: Progression model of heart failure towards the end of life.
Patients with HF tend to have a poor quality of life, especially those with a higher score in the New York Heart Association (NYHA) Functional Classification, weak socioeconomic status, and lack of social support.7-9 The benefits of palliative care (PC) are often forgotten. Such care, which goes far beyond symptomatic control, should be considered in an appropriate manner according to the patients’ needs.7,8,10 Given that we are facing an incurable and irreversible illness, there cannot be a rigid division between curative care and overall care designed to maximise comfort (Figure 2).8-12 This model balances the life-prolonging therapy with PC through most of the disease trajectory. When the active therapy is a viable option, minimal PC interventions are initiated. Once life-prolonging therapy becomes less of an alternative, PC becomes the primary method of clinical management.8-12 The proper palliation of symptoms should not be delayed until the last days or hours of life.13 The importance of PC and its inclusion in the therapeutic approach of HF is stated in the guidelines of the American Heart Association (AHA), the American College of Cardiology (ACC), the International Society of Heart and Lung Transplantation (ISHLT), and the European Heart Association (EHA).14-16
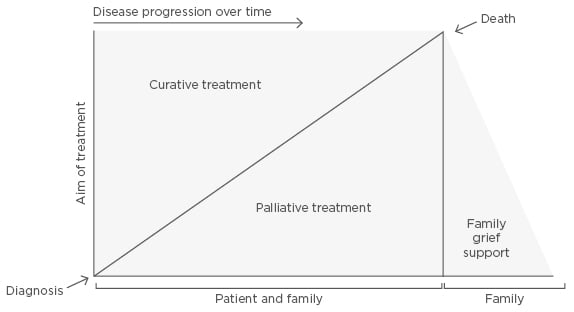
Figure 2: Multifactorial interactions between curative and palliative care.
There is compatibility between curative treatment, which permits life extension, and palliative care for symptomatic relief and quality of life; therefore, both approaches should be combined. Family/caregivers should also be included during the disease progress.
In the later stages of HF, it is important to identify patients with end-of-life HF (HF in the last 12 months of life) in order to offer appropriate care for the patients’ needs.17 Always acting on the basis of an acute care model, characterised by aggressive, disproportionate, and inefficient care, is not suitable in these clinical settings.18,19 Known scales, such as CARING or Gold Standard Framework, present global and specific deterioration indicators allowing the identification of patients with end-of-life HF.20,21
Cardiac cachexia (CC) is defined as the loss of >5% of body weight over 12 months in the presence of HF.21,22 This pathological entity affects around 5–15% of patients with HF and is generally present in NYHA III or IV functional classes. CC corresponds to a strong predictive factor of poor prognosis in HF, independent of other important variables, such as age, functional class, ejection fraction, and physical capacity, although it is related to them.7-9,22,23
CC pathophysiology is complex and multifactorial and, when fully established, it is hard to treat and reverse the process.21-26 The palliative approach to this class of non-oncological terminal patients has proven to be suboptimal.
The objective of this review is to gather evidence to correctly recognise CC and contribute to the improvement of clinical practice; namely, identification of early stages of muscle waste and sarcopenia, and better recognition of a patient with CC and terminal HF who is in need of PC due to their symptoms being no longer controlled by the usual medical measures.
PATHOPHYSIOLOGY OF CARDIAC CACHEXIA
Sarcopenia is defined as muscle wasting associated with functional impairment. It is characterised by a progressive and generalised loss of skeletal muscle mass in the limbs that exceeds two standard deviations of the mean of a healthy young reference and may be seen as a precursor of cachexia.20,21 Sarcopenia is found in 19.5% of patients with HF, and 68.0% of patients show muscle waste and reduced capillary density. If there is no intervention in cases of HF, there is a progressive loss of skeletal muscle mass and, in the latter stages, fat and bone mass loss leads to fully established CC.21-25 CC is present in 5–15% of advanced HF patients, and the mechanisms involved in the pathophysiology are multifactorial, involving reduced food intake, gastrointestinal malabsorption, neurohormonal disorders, overexpression of proinflammatory cytokines, increased oxidative stress, and an imbalance between anabolic and catabolic states.22-36
Reduced Food Intake
Several factors may be involved in the reduction of food intake, such as unsavoury diets due to low sodium content, severe depression, and visceral vascular congestion.29-31 Some drugs commonly used to treat HF may also be related to a reduced food intake; for example, captopril can cause palate changes; digitalis is sometimes responsible for anorexia and vomiting; and diuretics used in a vigorous way may lead to zinc and potassium depletion, which in turn reduces the intestinal motility and causes palate changes.15 The proinflammatory body status and the abnormal increase in serum levels of leptin and adiponectin are also responsible for anorexia. Early satiety due to hepatomegaly with gastric compression and the occurrence of dyspnoea at rest in NYHA Class IV functional class patients contribute to a reduced food intake.
Functional Modifications in the Gastrointestinal Tract
Vascular splanchnic congestion and collagen accumulation in the intestinal mucosa are typical findings in these patients. Such mucosal changes lead to a thickening of the gastrointestinal wall, reducing the number of intestinal villi and increasing the distance between the capillaries and the enterocytes. The accumulation of these modifications leads to intestinal malabsorption with a reduction in lipoprotein absorption.32 Additionally, there is an increasing concentration of the intestinal bacterial flora and higher adhesion of the biofilm to the sigmoid mucosa. Increased paracellular permeability leads to bacterial translocation with the release of endotoxins (lipopolysaccharides), which in turn stimulates the production of tumour necrosis factor (TNF)-α and other proinflammatory substances, contributing to a state of systemic inflammation.29-32
Neurohormonal Activation
In HF, activation of the sympathetic nervous system (SNS) occurs, raising the levels of noradrenaline and cortisol. This adrenergic stimulus promotes a cellular catabolic state and peripheral vasoconstriction that exacerbates splanchnic congestion. Permanent activation of the SNS leads to increased basal energy expenditure and activation of the renin-angiotensin-aldosterone system.32-34 As proven by animal models, angiotensin II, a significant mediator in CC development, induces muscle wastage by activating the ubiquitin-proteasome system (UPS), leading to apoptosis, a reduction in protein synthesis, and appetite impairment.28-34
Imbalance Between Anabolic and Catabolic Metabolism
The preservation and maintenance of skeletal muscle depends on the delicate balance between catabolic and anabolic mechanisms. The imbalance of these chemical processes forms the basis of the pathogenesis of sarcopenia and CC. The anabolic mediators are reduced, such as growth hormone, testosterone, insulin-like growth factor 1, ghrelin, and insulin. The major negative chemical processes concerned are the UPS, autophagy, apoptosis, inflammation, and oxidative stress.21-28 Proinflammatory cytokines, such as TNF-α, interleukin (IL)-1, IL-6, glucocorticoids, and adiponectin, play a cardinal role in muscle wastage by reducing the intracellular anabolic pathways. The activation of the UPS leads to lysosomal proteolysis by ubiquitination. Autophagy, a catabolic process that involves the lysosomal system, seems to be regulated by transcription factors (e.g. nuclear factor kappa B [NF-κB]), reactive oxygen species, and TNF-α. All of these elements lead to a disproportionate oxidative stress response, which in turn raises angiotensin II levels. It seems that the loss of mitochondria and mitochondrial dysfunction may also be implicated in the increase of cell-damaging oxygen free radicals.35-38
CLINICAL REPERCUSSIONS OF CARDIAC CACHEXIA
The clinical consequences of CC syndrome are related to muscle proteolysis, weight loss, and systemic inflammatory status, including changes in the cardiovascular and respiratory function; depletion of muscle mass due to atrophy, apoptosis, or necrosis, lowering the number of mitochondria and capillaries and increasing the predisposition for anaerobic metabolism with lactic acid production; impairment of urinary acidification and concentration; predisposition to pressure ulcers due to decreased healing capacity; gastrointestinal tract dysfunction; multifactorial anaemia due to nutrient malabsorption, systemic inflammatory status, iron deficiency and reduced erythropoiesis; and a decline in immunity, leading to a higher risk of infection. Due to the occurrence of these major metabolic alterations in CC, HF symptoms worsen.32-38
TREATMENT APPROACHES
It is difficult to establish a specific and effective therapy for CC syndrome due to its multifactorial pathogenesis. Physicians should be aware of muscle waste and sarcopenia even when the therapeutic options are effective and the full establishment of CC is delayed.
CC cannot be treated solely with an increase in nutritional uptake; exercise is also an important therapeutic approach. A combination of both strategies is recommended, including appropriate rehabilitation nutrition. Aerobic and resistance exercise training has the potential to reduce cytokine expression and increase anti-apoptotic factors, having an anti-inflammatory effect and improving functional capacity, therefore enhancing muscular regeneration.22,31,39-43 In patients with advanced HF, advanced age, or frailty, who are unable to tolerate daily aerobic and resistance exercise, neuromuscular electrical stimulation (NMES) might be an option.39,40 It has been demonstrated that NMES has the same anti-inflammatory properties as aerobic and resistance exercise training. In animal models, high frequency NMES (>50 Hz) induces an anabolic metabolic state due to an increase in glycolytic capacity, protein synthesis, expression of insulin-like growth factor 1, and muscle fibre size, which is also related to resistance training. Low frequency NMES (<20 Hz) has a similar activity to aerobic training exercise, inducing endurance and reducing autophagy.39,40 Although rehabilitation nutrition is of extreme importance, there is no dietary standardisation; it is characterised by an increase in protein uptake and an adequate vitamin supply of both soluble and lipo-soluble vitamins (vitamins A, D, E, and K).31,39-42
The pharmacological treatment approach for CC involves appetite stimulators, anti-inflammatory drugs, hormones, and anabolic stimulants. In the appetite stimulators category, treatment with megestrol acetate (160 mg twice daily) and L-carnitine (4 g per day) have both proven to increase body mass in clinical trials. Megestrol acetate is a derivative of progesterone widely used by oncologists, not only for the treatment of hormonal-related cancers but also as an appetite stimulant when appropriate.39-42
Since inflammation is a major contributor to sarcopenia, immunomodulatory and anti-inflammatory therapies were thought to be a logical option. Small clinical trials with pentoxifylline, thalidomide, methotrexate, and immunoglobulins showed no sustained benefit as pharmacological treatments.34,39,41 Clinical trials with beta-blockers demonstrated a delay in the development of CC and promoted a partial improvement in those with CC, since the drugs limit activation of the SNS.39,41,42
Ghrelin is a hormone produced by the stomach and acts on the pituitary gland to release growth hormone, which, in turn, reduces anorexia. It constrains the production of proinflammatory factors and induces the production of IL-10, a potent anti-inflammatory cytokine. Clinical trials demonstrated that ghrelin lead to an increase in body weight, body fat mass, and lean tissue mass, which ultimately permit appropriate exercise training.24,34,39,41
Anabolic steroids are effective at reverting and treating muscle wasting, although the risks associated with their administration surpass the possible benefits. Selective androgen receptor modulators have the same anabolic characteristics as treatments with testosterone, without the associated side effects on the skin, hair, and prostate. Enobosarm, an example of this new pharmacological drug class, has tissue-specific anabolic and androgenic activity, which improves lean muscle mass and physical function.24,34,36,39,41
In animal models, espindolol is a beta-blocker that increases body weight, lean tissue, and fat mass without affecting cardiac function, having a more favourable effect on preventing muscle waste than other beta-blockers. The ACT-ONE trial demonstrated these beneficial effects in cancer cachexia. The COPERNICUS trial proved that carvedilol reduced cachexia development and stimulated a partial reversal of cachexia in patients with severe HF.24,34,36,39,41,42
PALLIATIVE APPROACH
Discussing end-of-life issues with patients is challenging, especially with patients who often have a limited understanding of the nature and seriousness of their condition. Many patients defer to their clinicians for important decisions, choosing a more passive role.43-49
The PC approach is directed at improving the patient’s quality of life and addressing their family’s challenges related to their refractory symptoms. Routine comprehensive symptom assessment with validated instruments enables the prevention and relief of suffering and allows treatment of physical and psychological symptoms.8-12,16,17 Suitable acknowledgement of the shift from curative care to comfort care allows appropriate end-of-life care to be provided for both the patient and their family.8-12,16,17,43-49
When CC is fully established, it is fundamental to explain to caregivers and family members that clinical reversibility is less probable and emphasise the importance of the patient’s comfort.34,39 In these situations, the main medical practices that should be involved are general practice, internal medicine, cardiology, and palliative medicine. There should be a collaborative approach including physicians, nurses, therapists, psychologists, dietitians, social workers, and other health professionals to improve communication and understanding of the patient’s objectives to a have a better end-of-life.43-47 This methodology has shown to improve survival through patient education, including promotion of self-management skills, improving medication and dietary compliance, encouraging daily weighing and exercise, assuring close follow-up, and introducing end-of-life issues.8-10 Clear communication between the medical team, family members/caregivers, and the patient is vital to shared decision-making and various assumptions about the palliative approach should be demystified.2,8,10
The most frequent symptoms in advanced HF with CC are refractory dyspnoea, fatigue, anorexia, nausea, constipation, asthenia, and depression.44 Symptomatic palliation, which aims to reduce total suffering, comprises several strategies, both pharmacological and non-pharmacological. Non-pharmacological measures aim to help the patient adapt to the progressive losses that they will experience and include adjustments in the home, massages, acupuncture, and others.19,35
In the absence of a response to first line treatment efforts for refractory dyspnoea, including diuretics, post-load reduction drugs, and inotropes, progressive and titrated doses of morphine may be administrated for symptom relief, favouring the oral route.16-19 Non-pharmacological measures consist of directing fresh air towards the face with a fan, reducing the room temperature, opening windows, breathing humidified air, and elevating the head from the bed.35,43-50 Benzodiazepine may also be administrated to mitigate any associated panic symptoms.16-18
In the case of depressive symptoms, selective serotonin reuptake inhibitors may be used as a first therapeutic line option as they offer good efficacy and limited side effects. Behavioural therapy and psychotherapy also play relevant roles in symptom relief, as well as adapted exercises under specialised supervision.43-50
Common causes for nausea in advanced CC include a reaction to opioids and other medications, hepatic congestion, and ascites.43,44 Various antiemetic drugs with different mechanisms of action exist that can be used in situations of nausea, such as metoclopramide, haloperidol, first or second-generation antipsychotics (e.g. prochlorperazine and olanzapine), and serotonin agonists (e.g. ondansetron, granisetron). These pharmaceutical drugs can be used in combination to enhance their effects.22,43 In terminal HF, constipation is multifactorial and, in most situations, is related to dehydration, immobility, and side effects of certain drugs, but is generally overcome with laxatives or emollient drugs.43,48,50
In the end-of-life setting, glucocorticoids may be included in the symptomatic treatment of anorexia due to their role in temporarily increasing the appetite and energy.43 Regarding dehydration towards the end of life, clinical studies have demonstrated the lack of benefit of artificial hydration on patient quality of life or survival, with a high risk of worsening overload symptoms.51,52
In a study of ambulatory patients with various progressive organ failure diseases, around 60% of patients preferred discussions about their prognosis and future losses in the early stages of disease, rather than the last days of life, allowing adjustment of their expectations and acceptance.52 For a timely referral, the health professionals who care for these patients should be able to recognise indicators of the need for a palliative approach. United Kingdom Primary Health Care developed a model that allows an appropriate recognition of patients with chronic pathology in need of PC, named RADboud indicators for PAlliative Care (RADPAC), as shown in Table 1.49-53
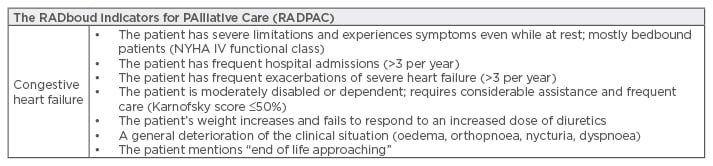
Table 1: RADboud Indicators for PAlliative Care (RADPAC).
NYHA: New York Heart Association.
As seen in many hospitals, patients with terminal HF and CC are treated in internal medicine wards, often without sufficient supportive care. In order to provide adequate care and attempt to prevent this syndrome, thus reducing its impact on healthcare, there should be a clearer communication between general practitioners, internal medicine physicians, cardiologists, and PC specialists since HF is a chronic and progressive disease with an unforeseeable course and is associated with an increasing number of deaths and different levels of suffering.
CONCLUSIONS
CC is a major prognosis factor of HF, influencing survival and quality of life. The pathophysiology is complex and multifactorial. There are many treatment approaches to prevent or reverse initial cases of CC. Nonetheless, physicians should be attentive and try to prevent this syndrome by having a multidisciplinary team including nutritionists, experts in rehabilitation, and experts in PC. In end-of-life situations, comfort should be the main concern and inadequate therapeutic measures that do not offer any benefit should be terminated.








