Interview Summary
Hypertrophic cardiomyopathy (HCM) is an inherited disorder that may cause disabling and potentially life-threatening symptoms related to thickening of the left ventricular wall. The impact of pathophysiological research on the treatment of HCM was central to the discussions with Gerald Carr-White, Deputy Medical Director of the Cardiovascular, Respiratory and Critical Care Unit at Guy’s and St Thomas’ NHS Foundation Trust, London, UK; and Michelle Michels, Head of the Center of Expertise for Inherited Cardiovascular Disease at the Erasmus University Medical Centre, Rotterdam, the Netherlands, during interviews conducted for the European Medical Journal (EMJ) in May and August 2024. The experts provided an overview of current options for the symptomatic treatment of HCM, including the advent of targeted therapies, cardiac myosin inhibitors (CMI), for the obstructive form of the condition. They explored the advantages and limitations of current therapies and shared their informed opinions on how precision medicine and genetic therapies have the potential to transform patient care. While highlighting the treatment of non-obstructive disease as a significant unmet need, Carr-White and Michels expressed optimism for the future of HCM treatment, driven by an understanding of the underlying pathophysiology and guided by increasing clinical and real-world evidence.INTRODUCTION
HCM is an inherited heart condition characterised by a thickening of the left ventricular wall that may be obstructive (oHCM) or non-obstructive (nHCM) to blood flow from the heart. The effects of HCM can range from mild or even asymptomatic, to severely disabling with risk of sudden cardiac death (SCD). However, until recently, pharmacological treatment of HCM was based solely on existing therapies for controlling arrhythmia and blood pressure, such as beta blockers and calcium channel blockers. In this interview article, cardiology specialists, Michelle Michels and Gerald Carr-White, share their views on how advances in pathophysiological research have led to targeted pharmacotherapies developed specifically for HCM, shaping the future of patient care.
Michels provided an overview of the condition: “HCM is characterised by left ventricular hypertrophy that is not explained by known loading conditions such as hypertension or aortic valve disease. In adults, HCM is defined by an increased wall thickness of ≥15 mm, and most patients will have some kind of obstruction [denoted by] a pressure gradient in the left ventricular outflow tract (LVOT) of ≥30 mmHg.” Both experts emphasised the importance of actively searching for an obstruction by performing a Valsalva manoeuvre and/or exercise during the assessment. “If you use provocation during your echocardiography, about 75% of patients will have some form of obstruction,” Michels noted, with the remaining patients designated as having nHCM.
Discussing the aetiology of HCM, Michels explained, “We’re able to identify likely pathogenic or pathogenic gene variants in about half the patients, and most of these are in the sarcomere (the smallest contractile unit of the heart). However, there are also cases of HCM without an identified gene variant.” Carr-White concurred, stating, “Our genetic understanding is changing. Quite a lot of patients with HCM have a monogenic ‘spelling mistake’ in one of the genes that tend to pass through families in an autosomal dominant pattern; but we’re also starting to see some cases without the common HCM genes, so it could be more of a polygenic inheritance with several different genes interacting with environmental factors.”
Carr-White also noted that HCM is also more common than generally realised: “Most people with HCM probably remain undiagnosed, but we think about one in 300 or 400 people have HCM.” Michels added, “If you also take into account the prevalence of unaffected gene carriers, so those who carry a likely pathogenic or pathogenic gene but without hypertrophy, it might be as high as one in 200 people.”1
Presentation: The Symptoms and Impact of HCM
Although HCM has considerable clinical heterogeneity, core symptoms were described as breathlessness, palpitations, and/or dizziness on exertion, which may be accompanied by chest pain. There is also an association with cardiac arrest and SCD; however, the experts emphasised that SCD was rare as the first presentation of HCM. At the other end of the scale, Michels also pointed out that HCM can be asymptomatic and so may be diagnosed incidentally during a physical examination, electrocardiogram, or family screening.
Regarding the impact on daily life, Carr-White explained, “It’s quite a spectrum, but there’s a reasonably large group (probably about 20% of patients, more often those with oHCM), who can have quite disabling symptoms with very marked chest pain, dizziness, breathlessness, and syncope at times. However, there are very effective treatments for symptomatic LVOT obstruction (as summarised below). With the armoury of treatments currently available, you can hope to significantly improve patients’ symptomology in the vast majority of cases.”
Pathophysiology of HCM
A more detailed understanding of the pathophysiology underlying HCM has, in recent years, been pivotal in directing the development of treatments specifically for HCM. Carr-White summarised: “In most monogenic HCM disorders, you see ‘spelling mistakes’ in the genes for proteins that sit within the cardiac myocyte, particularly within the sarcomere. Most of these genetic changes affect the myosin–actin cross bridges and the contractility of how the cell beats. This creates overactivation, as you often get more of these cross bridges, and so more contractile function at the cellular level. Over time, this leads to hypertrophy of the muscle and fibrosis. This myocyte disarray is one of the characteristic features observed under the microscope.” Michels emphasised, “What is very important in HCM is that the heart muscle becomes stiffer, so its ability to relax is also hampered by the increased number of cross-bridges.”
According to Carr-White, the impact of these pathophysiological insights has been significant: “Understanding the pathophysiology of HCM has helped to develop a group of directly acting myosin inhibitors, which target the actual pathophysiology underlying HCM. I think that’s been a great advance.” Michels agreed, “This knowledge has led to new insights and new therapies, and is altering the treatment options for patients with HCM.”
Symptomatic Treatment Strategies
The experts went on to describe the rapidly evolving treatment landscape for HCM. Carr-White commented, “The first thing to do is improve the patient’s symptoms, considering medications or, if necessary, more invasive therapies.” Michels recommended a recent review by Hutt and Desai2 as an excellent summary of current medical treatment strategies, and Carr-White highlighted the 2023 European Society of Cardiology (ESC) cardiomyopathy guidelines as a valuable new resource,3 being the first European guidance on the overall management of cardiomyopathies (a specific guideline for HCM had been published a decade earlier in 2014).4 The USA Guideline for the management of HCM was also revised in 2024.5
The experts described the European and USA guidelines as similar in terms of recommendations for the symptomatic treatment of HCM. After general measures such as weight loss, the avoidance of dehydration, and reducing excess alcohol consumption, both guidelines recommend either beta blockers or calcium channel blockers (verapamil, diltiazem) as first-line treatments for obstructive disease.3,5 If patients are unable to tolerate these first-line therapies, have contraindications, or don’t respond, the European guidelines recommend adding disopyramide (a sodium channel blocker) or mavacamten (a cardiac myosin inhibitor [CMI], in adults only; Figure 1). If this also fails, then alcohol septal ablation or surgical myectomy are further options.3 “The only slight difference in the USA guidelines is their second-line puts septal ablation, mavacamten, and disopyramide all in parallel,”5 said Carr-White. Michel described CMIs as the ‘new kids on the block’, recommended by both the European and USA guidelines for the treatment of oHCM: “Now we have CMIs as the next step if the first-line medical treatment fails, whereas previously the next step would have been septal reduction therapy. This makes a huge difference for patients.”
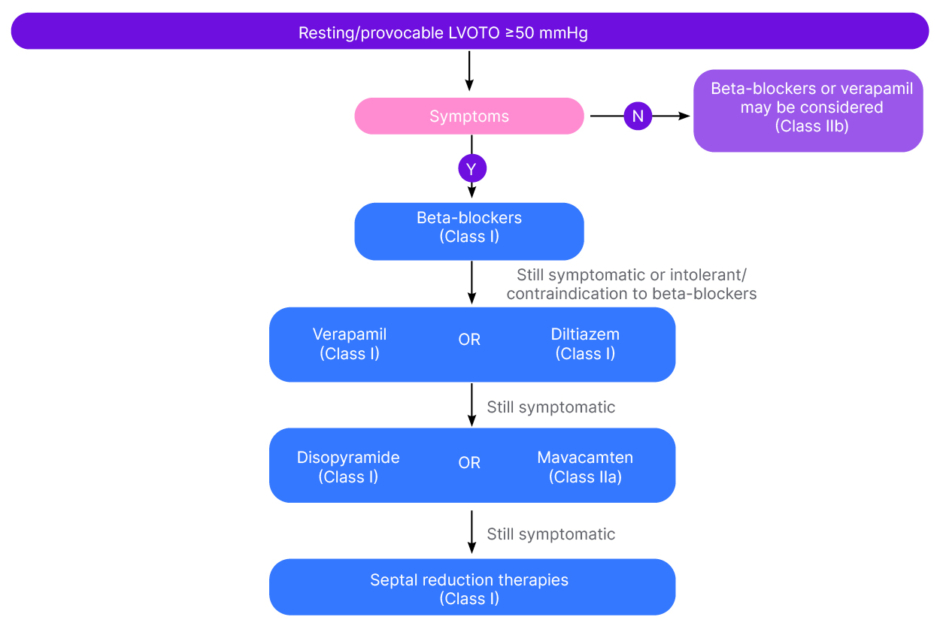
Figure 1: Flow chart on the management of left ventricular outflow tract obstruction (European guidelines).3
LVOTO: left ventricular outflow tract obstruction; N: no; Y: yes.
Regarding non-obstructive disease, Carr-White commented, “Treating patients with nHCM tends to be more difficult. [The advice is] to try calcium antagonists, beta blockers, sometimes low-dose diuretics, and occasionally a small dose of nitrates.” Clinical studies of the CMIs for nHCM are currently ongoing.6,7 Overall, the USA guidelines describe symptomatic nHCM as “a diagnostic and therapeutic challenge” due to differences in disease onset, severity, and risk of adverse outcomes.5
First-Line Pharmacotherapies: Beta Blockers and Calcium Channel Blockers
Current first-line therapies for HCM have been the foundation of care for many decades, developed in what Michels described as “a different era” in terms of clinical study.8 The experts emphasised the value of extensive real-world clinical experience with these drugs, while also noting the limited number of supportive pathophysiological or clinical studies specific to HCM.
Referring to obstructive disease, Carr-White commented, “In day-to-day practice, we’ve all used beta blockers for decades, and treatment definitely does improve patients’ symptoms. However, in terms of scientific evidence, there are very few good clinical trials in HCM. There are some trials showing that beta blockers reduce the outflow tract gradients, but these are quite small (certainly nothing along the lines of the CMI trials we’ve seen recently).” Michels added, “We believe the mechanism of action is that diastolic pressure is reduced and left ventricular filling is improved. Small clinical trials, including, most recently, a placebo-controlled, 2-week study of metoprolol,9 observational cohort trials, and clinical experience have led to beta blockers becoming the first line of treatment in oHCM. Beta blockers followed by calcium channel blockers, which have also been tested in small groups of patients, have been used for a long time in HCM, and in general are readily available and quite easy to use.”
Regarding safety, Carr-White described beta-blockers and calcium channel blockers as having a generally favourable profile, established over decades of use in various cardiological conditions: “I think they’re safe drugs, and most people tolerate them reasonably well. However, quite a few people struggle with beta blockers; they often get cold extremities, fatigue, erectile problems, and poor sleep.” Michels expanded on this point, explaining that beta blockers are unsuitable for patients with bradycardia, low blood pressure, or certain pulmonary diseases, due to their slowing effect on the heart. A specific caution was also raised about the risk of pulmonary oedema with calcium channel blockers in patients with high LVOT gradients.
Second-Line Pharmacotherapies: Disopyramide
The addition of disopyramide is a treatment option for oHCM in patients who do not respond to or tolerate first-line therapies.3,5 Michels described it as “a negative inotropic drug without a vasodilator effect, which can be very helpful in oHCM. However, there are important side effects, especially anticholinergic ones, and it can’t be combined with other drugs that also prolong the QT interval. It can also be a problem for patients with glaucoma or males with prostatism.” Based on their clinical experience, both experts also highlighted difficulties with drug availability. “Certainly, in the UK, it is hard to get hold of disopyramide, so the vast majority of physicians would consider mavacamten [as their second-line therapy],” said Carr-White.
Targeted Pharmacotherapies: Cardiac Myosin Inhibitors
The arrival of targeted therapies in the form of CMIs has been a notable development for the treatment of oHCM.10 The CMIs mavacamten and aficamten reversibly bind to cardiac myosin ATPase at two distinct allosteric sites, inhibiting its activity and thereby reducing actin–myosin cross-bridge formation.6,11 Carr-White explained, “We’re very excited by these new drugs. They target the actual pathophysiology underlying HCM: the hyper-contractility due to the increased number of actin–myosin bridges.” Michels shared this enthusiasm: “We know that mavacamten binds specifically to the myosin head.12 It’s a very small molecule, and it reduces the number of cross-bridges to the level seen in those without HCM.6,12 These are impressive data. It’s working on the known changes in the sarcomere.”
Currently, mavacamten is the only CMI approved in the USA and Europe,13-15 with aficamten likely to be the next-in-class.3,5 Michels summarised, “There are three important Phase III trials: the EXPLORER-HCM trial and the VALOR-HCM trial,16-18 both double-blind, placebo-controlled trials of mavacamten in oHCM, and both very positive in the primary and secondary endpoints [of improved exercise capacity, LVOT obstruction, New York Heart Association [NYHA] functional class, and health status]. Then, this year, we had the results of the SEQUOIA-HCM trial of aficamten in patients with symptomatic oHCM,19 which was also double-blind and placebo-controlled, and demonstrated very good efficacy (reaching primary and all secondary endpoints), as well as safety. This is a great difference compared to the other drugs that we use in HCM, because these are really large, placebo-controlled randomised trials, something we haven’t seen before.” Carr-White reinforced this point: “The trials have shown very clearly that CMIs reduce LVOT obstruction, and the associated symptoms, considerably.”
Regarding safety, Carr-White observed, “The side-effect profile seems favourable based on the limited number of patients we’ve treated, as well as the trial data and real-world data, particularly in the USA. The only thing to watch for is the small risk of worsening left ventricular dysfunction.” Michels continued, “Because they influence systolic function, these drugs are only suitable for patients who still have a preserved ejection fraction (although most patients with oHCM do have a high ejection fraction). Also, mavacamten can’t be used in females of childbearing age who are not using effective contraception due to its potential effect on the fetus.13,14 There are drug–drug interactions, though in my experience we have always been able to adjust the background medication.”
In terms of practicalities, both experts highlighted frequent echocardiographic monitoring for left ventricular dysfunction as a current limitation of using the approved therapy, mavacamten. “In order to use mavacamten, there’s a very strict protocol to follow, including many echocardiograms.13,14 This puts a burden on healthcare resources,” said Michels, and Carr-White agreed, “I hope as we gain greater experience, we may be able to find a more personalised way to monitor for left ventricular dysfunction.”
Overall, the experts’ opinion on the prospects for these new targeted therapies in HCM was strongly positive. “They’re a very exciting treatment, and I think they will be used more and more,” said Carr-White. “We’re entering the era of precision medicine for HCM. If you look at the trials, the real-world data, and also our own experience, the majority of patients really improve,” observed Michels.
UNMET NEEDS AND FUTURE PERSPECTIVES
Treatment Developments
As described above, the advent of targeted therapies may frame an encouraging future for the medical management of HCM. Both experts noted the anticipated arrival of aficamten as the next-in-class CMI, based on positive Phase III clinical data from the SEQUOIA-HCM trial.19 Within the same CMI class, mavacamten and aficamten exhibit overlapping but distinct profiles.11 “A notable difference is that aficamten has a shorter half-life [than mavacamten], which makes it a little easier to dose in patients,” said Michels. “Also, mavacamten is metabolised through cytochrome P450, which is why you see drug–drug interactions that you don’t see with aficamten. These are two important differences, just in pharmacokinetics.” In addition, Michels expressed interest in the ongoing Phase III MAPLE-HCM study (NCT05767346) which compares aficamten head-to-head with the beta blocker, metoprolol, in patients with symptomatic oHCM.20 This investigation is particularly significant, as the lack of a head-to-head comparison has, to date, prevented the recommendation of CMIs as a first-line therapy, according to the European guidelines.3
Michels also highlighted recent research into metabolic and gene therapies: “There have been developments in metabolic therapy, including a Phase II trial with ninerafaxstat in non-obstructive disease, but we have to wait for the Phase III trial.21 Gene therapy is also being studied. It’s only in Phase I, but I think it could be very promising.” Carr-White added that the USA Cleveland Clinic had enrolled the first patient in the Phase Ib gene therapy (MyPeak-1) trial for nHCM,22 and stated, “I think that gene-modifying replacement therapy is going to really expand over the next 5 years, and that will be very exciting.”
Unmet Needs
Michels and Carr-White discussed what they viewed as the key unmet needs for the symptomatic treatment of HCM, highlighting the development of therapies for nHCM as a priority. “We have seen a lot of recent developments in patients with oHCM but a quarter of patients have nHCM, and currently, we don’t have any specific therapy for this group, who can be severely symptomatic. So, it’s good that CMIs, mavacamten and aficamten, are currently being studied in Phase III trials for nHCM (ACACIA-HCM [NCT06081894]; ODYSSEY-HCM [NCT05582395]),6,7,23,24 said Michels, who also spoke about the need for real-world evidence to further guide the use of CMIs: “Mavacamten has been available here in the Netherlands for just 5 months, so we need real-world data on both the efficacy and safety of the drug. This might help to reduce the number of echocardiograms. It would also be helpful to be able to predict which patients won’t respond to the treatment. That’s very important in precision medicine. There is also an unmet need for treating HCM with a reduced ejection fraction, as CMIs cannot be used in these patients.”
Carr-White outlined further unmet needs in patient management: “The biggest problem in the UK is the increasing number of patients as we improve diagnosis and screening. It’s not uncommon for patients with HCM, a potentially lethal condition, to wait 12 months or longer to be seen. Therefore, I think that the priority here is to develop functional ‘hub and spoke’ networks, and free up space within the system to fast-track those who need treatment the most: the symptomatic and high-risk patients.” Carr-White also addressed the urgent need to improve psychological services for patients with HCM: “They are often a quite vulnerable group, particularly when worried about the risk of sudden death and passing the condition on to their children. Cardiologists tend to focus on the cardiac side of things, whereas genetics nurses or counsellors may be better at addressing psychological concerns. Also, if a physician has limited experience with HCM, it’s important to embed them within a wider team and direct them to think about three aspects: treating symptoms (highlighting new treatments); assessing the risk of sudden death (annually); and genetic testing the wider family.”
Future Perspectives
Looking to the future, the experts emphasised the importance of further elucidating the cardiac pathophysiology in HCM. Carr-White predicted, “It will be an iterative process, getting more precise, targeted molecules as we gain a greater understanding of the pathophysiology. Also, going forward, we hope that because [a drug] targets the underlying pathophysiology, it may be able to target the natural progression of the condition.” Michels agreed, “Is it beneficial to treat earlier? Do you only treat symptoms, or do you also treat to prevent problems from happening in the future?”
Carr-White speculated, “We may get more personalised medicine with different approaches to particular genes,” and Michels noted that, “the CMIs were developed based on HCM caused by a specific underlying gene variant. However, in the clinical trials, we give them to every patient with the oHCM phenotype, and both groups respond. So that’s intriguing.” Michels also highlighted the importance of being able to predict how the phenotype will develop for likely pathogenic or pathogenic gene carriers but noted the difficulty of this process: “There’s extreme clinical heterogeneity, even among patients carrying the same background gene, so I think it’s important to look also at ‘second hits’; for example, obesity and hypertension. I think that’s vital for the patients. It’s also critical to phenotype your patients well, because phenocopies like cardiac amyloidosis or Fabry’s disease have their own specific treatments. That’s the flip side of the coin [of precision medicine].”
Looking ahead to future clinical study programmes, Carr-White suggested, “We need to look at harder outcomes such as SCD and prognosis, which we’ve never really examined in HCM. This will involve multicentre trials, or large registries, across Europe, or Europe and the USA. I think that’s the next level of research, which will be very exciting but will come with quite a few challenges.”
CONCLUSIONS
Overall, the experts were highly optimistic about the future of treatment for HCM, with further developments expected in targeted treatments based on pathophysiological and genetic findings, and a greater volume of robust clinical data and real-world evidence. Carr-White commented, “Drugs are coming through the system that will target slightly different parts of the sarcomere, and numerous different genetic therapies are also being trialled. I think that combination of approaches will give us plenty of opportunities.” “I see many patients with HCM,” said Michels, “and most are really excited about these new developments. I think the landmark change of the CMIs is really promising. It’s very good that most patients do improve, and I believe it’s important, when you see patients, to stay motivated to try and improve their lives. I think the future looks bright for HCM.”
Biographies
Gerald Carr-White
Deputy Medical Director of the Cardiovascular, Respiratory and Critical Care Unit, Guy’s and St Thomas’ NHS Foundation Trust, London.
Michelle Michels
Head of the Centre of Expertise for Inherited Cardiovascular Disease, Erasmus University Medical Centre, Rotterdam, the Netherlands.







