Meeting Summary
The symposium was held on the final day of the European Academy of Allergy and Clinical Immunology’s (EAACI) Food Allergy and Anaphylaxis Meeting & European Consortium on Application of Flow Cytometry in Allergy (FAAM-EUROBAT) meeting in Athens, Greece. The discussions focused on the unmet labelling needs of prepackaged foods for patients with allergies, the regulatory changes under consideration for precautionary allergen labelling (PAL), and the variability in peptide profiles among extensively hydrolysed formulas (EHF) used in managing cow’s milk protein allergies.
The use of voluntary PAL and its benefits and drawbacks were discussed froma patient’s perspective, including how PAL affects their ability to manage their food allergy and what improvements they feel are needed.
This was followed by a presentation on the international regulations regarding food allergen labelling, which included recommendations from the Food and Agriculture Organization of the United Nations (FAO) and WHO expert consultation, as well as the latest draft Codex Alimentarius Committee on Food Labelling (‘Codex’) guidelines concerning risk assessment and labelling of unintended allergen presence. An example of what this will mean for the consumer when precautionary allergen labelling is aligned with the Codex draft proposal on allergen risk assessment was given, and the main challenges were discussed.
Lastly, the heterogeneity of EHFs available on the market for the management of cow’s milk allergy was emphasised, with a focus on the relevance this has to the allergenicity of the product. The potential benefits of introducing more stringent standards for these products in order to better address the needs of infants with cow’s milk allergy was considered.
The Patient’s Perspective on Precautionary Allergen Labelling
Sabine Schnadt, a specialist allergy dietitian at the German Allergy and Asthma Association (DAAB), Mönchengladbach, discussed how PAL affects the ability of patients to manage their food allergy, and how patients feel the medical profession and/or food industry could better support them.
Allergen information provided on prepackaged food is intended to provide patients with the necessary information they need to make informed and safe food choices.1 To achieve this, it should be clear and unambiguous.1 Schnadt explained that allergen information should ideally be presented at three levels:
- Ingredient list (mandatory): indicates a hazard.
- PAL (voluntary): indicates an assessable risk that an allergen may be present.
- No PAL (voluntary): indicates that a food has a low/no risk of inducing an objective allergic reaction in the majority of sensitive consumers.
However, Schnadt pointed out that, currently, patients feel uncertainty about PAL.2 Since PAL is a voluntary statement, a food without PAL cannot automatically be assumed to be suitable for patients with food allergy.
A prospective cohort study conducted among 157 adults who had a physician-confirmed diagnosis of IgE-mediated food allergy in the Netherlands found that, out of 51 foods that caused allergic reactions in these adults over the course of a year, food allergens could be analytically detected in 19/51 foods. Ten of these foods did not have a PAL statement on the label (and did not contain the eliciting allergens as ingredients). Therefore, there was no indication to the patient that this food could be a risk, and thus no opportunity for the patients to protect themselves. Of the remaining nine foods that carried a PAL, four contained allergens not specified in the PAL statement, showing that even if PAL is used, the statements are not always complete.3
Schnadt concluded that whether or not PAL is included on a prepackaged food, the only certainty is that there is a non-assessable risk that an allergen may be present. Because the presence or absence of PAL conveys ambiguous information without a clear message, she feels that consumers with food allergies are currently unable to make informed and safe food choices.1,4 An example of helpful and unhelpful scenarios regarding the presence or absence of PAL can be seen in Figure 1.
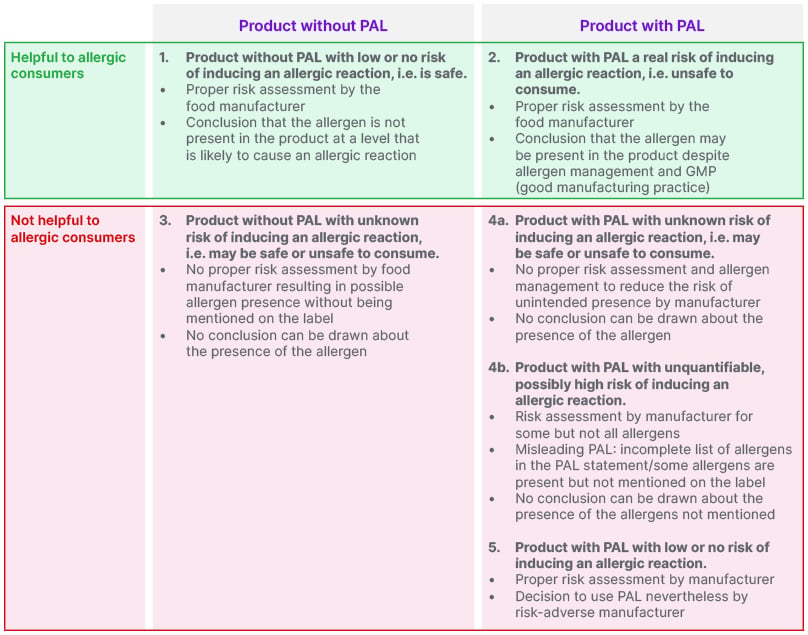
Figure 1: Scenarios for the presence or absence of precautionary allergen labelling (PAL).5
In a consumer survey by the DAAB, the vast majority of patients with peanut and/or hazelnut allergy felt that legal regulation of mandatory PAL would be one of the most useful measures/frameworks to help them manage their food allergies (DAAB survey 2023, unpublished data).
One of the difficulties in assessing the risk of an allergic reaction to a food product is the individual nature of the eliciting dose (ED) of an allergen, which is the amount of an allergen at which a patient experiences an objective allergic reaction.6 ED varies between patients, for example, a patient with a peanut allergy with an ED of 3 mg might expect to experience an allergic reaction when consuming a biscuit assumed to contain peanut residue on approximately 30% of occasions. In contrast, a patient with an ED of 300 mg might expect to experience an allergic reaction on only 0.012% of occasions.7
Patients on the higher end of the ED range are therefore more likely to tolerate trace levels of unintended peanut protein that may occasionally be found in prepackaged food products. It has also been shown that if these patients are allowed to ignore PAL based on their medical history or results of oral food challenges, they may experience a significant improvement in their quality of life.8
Schnadt summarised the changes that patients with food allergies need in terms of PAL as follows (Schnadt, personal communication):
- A regulated framework for meaningful PAL, based on quantitative risk assessment and reference doses that are relevant to health and safe for the vast majority of patients with food allergies.
- Personalised healthcare with improved diagnostics for food allergies, and options for therapy and management that take into account individual conditions, including ED.
Precautionary Allergen Labelling: International Regulatory Perspective
Marie-Claude Robert, Research and Development Expert for Allergen Management at the Institute of Food Safety & Analytical Science at Nestlé Research, Lausanne, Switzerland, described the latest international regulatory guidelines for PAL and what this will mean for the consumer when the food industry aligns with them.
The Codex is a collection of internationally adopted food standards overseen by the Codex Alimentarius Commission, which is part of the FAO/WHO food standard programme.5,9,10 Today, the commission has 188 member countries and one member organisation (the EU).10 In 2019, the FAO and WHO convened an expert consultation to address the issue of inconsistent and unregulated PAL use, the outputs from which are being considered by the Codex Committee on Food Labelling (CCFL) for implementation into the Codex.1,5,11,12
One of the recommendations from the expert consultation, published in 2022, is that the priority food allergens should be updated to: cereal containing gluten, crustacean, egg, fish, peanut, milk, tree nuts (specifically hazelnut, cashew, walnut, pistachio, pecan, and almond), and sesame.11 Robert stressed the importance of producing a harmonised list of priority allergens and the use of this list in national recommendations to improve safety for patients with allergies, particularly when they travel.
In a draft annex to the Codex, the CCFL specifies that allergen-management practices (e.g., controls to prevent/minimise unintended presence of food allergens via cross-contamination) should be implemented by a food-producing company before deciding whether to use PAL.9 Once these practices have been applied, the decision should be based on the findings of a risk assessment regarding the unintended presence of food allergens.9 PAL should be used when the presence of the allergen cannot be mitigated to at, or below, the action level, defined as the reference dose of the allergen (mg total proteins from the allergen) divided by the amount of food (kg) that can reasonably be expected to be consumed on a single eating occasion (preferably using the 50th percentile [P50]).9 While Codex members agree that the use of PAL is mandatory when exceeding action levels, discussions are still ongoing regarding whether PAL could be used voluntarily at doses below the action level of an allergen to ensure increased protection for particularly sensitive populations.9
Recommended reference doses were previously based on levels expected to protect 99% of the allergic population (ED01); however, the FAO/WHO now recommend doses that are expected to protect 95% of the allergic population (ED05).1,9 The rationale behind this change is that among patients who do develop symptoms to a food product with PAL, the probability of anaphylaxis is very low (<5%).1 This was illustrated by Patel et al.13 who explained that with a 99% protective level, 0.04% of individuals with a relevant allergy would react with anaphylaxis, and with a 95% protective level, 0.24% would react with anaphylaxis. Robert emphasised that a reference dose for gluten-containing cereals, including barley and rye, still needs to be established by the FAO/WHO Expert Consultation members.
In terms of communicating PAL to consumers, the CCFL recommend that a PAL should appear as a separate statement directly under, or in close proximity to, the ingredient list (when present); should start with ‘may contain’ (or equivalent words) and include the identified allergenic food(s) using the Codex-specified names for the foods and ingredients; and should contrast distinctly from surrounding text.9
Robert described several challenges in developing updated, internationally recognised Codex guidelines regarding PAL, namely:
- Reaching a consensus among Codex members on whether to use ED01- or ED05-based reference doses for determining action levels for each of the priority allergens.9
- Achieving agreement on the appropriate percentile value to determine the reference amount of a food consumed in a single eating occasion (e.g., 50th [P50] or 75th [P75] percentile).12
- Establishing a reference dose for gluten-containing cereals, including rye and barley.9
- Ensuring small-to-medium enterprises and developing countries can adopt and implement the new Codex guidelines.6,9
- Identifying and recommending suitable performance-tested and validated analytical methods, while ensuring that the reporting units for these analytical methods are expressed as [allergen] proteins per kg.9
She also emphasised the importance of considering whether differentiated labelling or specific claims could be applied to products designed for individuals who are highly sensitive to an allergen. It will also be crucial to communicate clearly to consumers when these legislative changes will be implemented, as this may result in PAL changes for a product without necessarily indicating a reduction in allergen content due to cross-contamination. Ultimately, all efforts to harmonise risk assessment and allergen labelling should contribute to restoring confidence in PAL.
Robert stressed two final points. First, it is essential to understand what a PAL is intended to communicate to consumers; namely, the risk of experiencing severe and potentially life-threatening reactions. She explained that the new draft expert recommendations for PAL are aimed at protecting 95% of the allergic population and that it is important to communicate this effectively to patients. Second, while she believes that larger industries are prepared to adopt new standards and PAL guidelines once they form part of the Codex, the more significant issue is the need for internationally harmonised regulations and thresholds. If countries do not adopt harmonised approaches to allergen labelling and PAL, the same food products with identical unintended allergen content could be sold in different markets while carrying different PALs. This discrepancy could mislead consumers, put sensitive individuals at risk, and ultimately undermine the objective of restoring confidence in PAL (Robert, personal communication).
Towards Setting Standards for Extensively Hydrolysed Formula
Clare Mills, Professor of Food and Molecular Immunology at the University of Surrey, UK, discussed the clinically relevant allergens present in cow’s milk and the allergenicity of extensively hydrolysed formula (EHF), with a view towards setting new standards for these formulas.
The most clinically relevant cow’s milk allergens are casein proteins and whey proteins (α-lactalbumin and β-lactoglobulin; Figure 2).14 Caseins constitute ~80% of the protein fraction of cow’s milk.15,16 They assemble into micelles, encasing clusters of calcium phosphate, allowing cow’s milk to provide around 10 times more calcium than would be possible in a simple aqueous solution.15,17 In the whey fraction of cow’s milk, β-lactoglobulin accounts for 50% of the protein, with α-lactalbumin the second-most common.16 Patients with cow’s milk allergy all develop IgE to casein with many also being sensitised to whey proteins.16
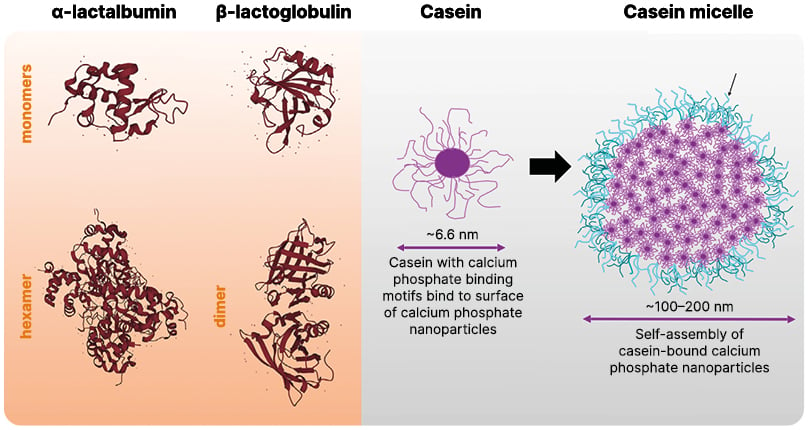
Figure 2: The major molecular milk allergens.
Adapted from Jensen SA et al.18 2022 and Holt C.15 2021.
In terms of their IgE-binding capacity, these proteins comprise both conformational (discontinuous) and linear (continuous) IgE-binding sites, also known as epitopes.16 Food processing procedures, such as heating, can modify the allergy-eliciting capacity of cow’s milk allergens by disrupting these epitopes and preventing IgE-binding. Heat treatment, however, can only modify conformational epitopes, and has little to no effect on the allergenic potential of cow’s milk proteins, particularly caseins.16 Hydrolysis, on the other hand, applies chemical or enzymatic methods to break down the polypeptide chains in proteins,16 and as such, will disrupt both conformational and linear epitopes. This approach, followed by further processing, is used to reduce the allergenicity of cow’s milk when producing formula.16
Two types of hydrolysed products are used in infant formula manufacture, producing EHF or partially hydrolysed formula (PHF). Although there is no international consensus for the definition, PHF is comprised of peptides with a molecular weight generally <5 kDa, while EHF is comprised of peptides <3 KDa.19 Studies have suggested that partially hydrolysed proteins may retain intact IgE epitopes. For example, when purified β-casein was digested into several fragments by plasmin, it retained its IgE-reactivity.20 Similarly, when whey protein isolate was hydrolysed by trypsin and chymotrypsin to degree-of-hydrolysis values of 18.7%, 22.5%, and 27.1%, IgE-binding capacity was reduced by 58%, 69%, and 73%, respectively, but not eliminated.21
Extensive hydrolysis reduces proteins to much smaller peptides than partial hydrolysis: an analysis of extensively hydrolysed whey protein and extensively hydrolysed β-lactoglobulin showed that they contained peptides of sizes up to 2.5 kDa and 3.5 kDa, respectively, but that >75% of the peptides were between 0.5–1.5 kDa.22 However, gel permeation chromatography of hydrolysates from the same study found that >55% of whey protein peptides aggregated into complexes of up to 20 kDa, and >40% of the β-lactoglobulin peptides aggregated into complexes of up to 25 kDa.22 Since this raises the question of whether these larger complexes are able to elicit an allergic IgE response, the hydrolysates were assessed for immunogenicity in the Brown Norway rat model, which demonstrated that they retained no sensitising capacity and no residual allergenicity.22
A study of the effect of hydrolysis in commercial infant formulas published in 2000 demonstrated that, at that time, some formulas described as partially or extensively hydrolysed contained protein material with an apparent molecular weight well above 3–5 kDa.23 The authors of the study reported the range of peptide sizes were more dependent on the manufacturer than on the reported degree of hydrolysis or the protein source.23 Perhaps because of these residual larger peptides in infant formula, there were concerns at this time about overreactions in highly sensitised children with cow’s milk allergy.24
Mills emphasised that there have been substantial advances in this field since that study was published, including the availability of some improved tools for processing infant formula and monitoring quality. However, a study conducted more recently, in 2020, showed that there remains considerable variability between infant formulas in terms of the sizes of peptides they contain (Figure 3), with some formulas that contained larger peptides also demonstrating in vitro IgE reactivity.25
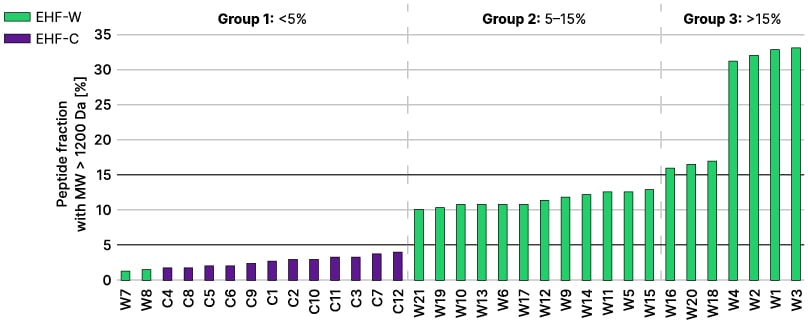
Figure 3: Percentage of peptides with a molecular weight >1.2 kDa in extensively hydrolysed formula.25
EHF-W and EHF-C products are depicted in green and purple bars, respectively. The black lines represent the 5% and 15% threshold. Three EHF groups were identified according to the fraction of peptides with a molecular weight >1.2 kDa.
EHF-C: casein-based extensively hydrolysed formula; EHF-W: whey-based extensively hydrolysed formula.
This issue of heterogeneity between hydrolysed infant formulas suggests that there may be benefits to setting new standards for these products.25 However, Mills explained that this would first require some of the terms used in this field, such as ‘hypoallergenic’, ‘partially hydrolysed’, and ‘extensively hydrolysed’ to be defined in clinically relevant terms to ensure consistency across manufacturers. She also stressed that chemical composition (e.g., peptide size distribution and residual IgE epitope sequences) should be linked to clinical reactivity. Finally, Mills emphasised that any discussion around hydrolysed infant formula standards should involve multiple stakeholders, such as patients, clinicians, manufacturers, medical societies, regulators, and experts.
Conclusion
The presence or absence of PAL on prepackaged food currently conveys ambiguous information without a clear message, meaning that consumers with food allergy are not able to make informed and safe food choices.1,4 Most patients feel that legal regulation of mandatory PAL would be one of the most useful measures to help them in living with their food allergy (Unpublished Data, DAAB Survey, 2023).
Expert recommendations to address this issue are being considered by the CCFL for implementation into the Codex.1,5,11,12 However, challenges include reaching a consensus on the reference doses and action levels for each allergen,9,12 establishing a reference dose for gluten-containing cereals (including rye and barley),9 achieving agreement on the appropriate percentile value to determine the reference amount of a food consumed in a single eating occasion, selecting and recommending performance-tested and validated analytical methods,9 and involving small-to-medium enterprises and developing countries in the harmonised adoption and implementation of such standards.6,9
There remains considerable variability between extensively hydrolysed infant formulas (recommended for management of cow’s milk protein allergy) in terms of the sizes of peptides they contain and their IgE allergenicity,25 suggesting that there may be value to setting new standards for these products,25 including clinically relevant definitions for terms such as ‘hypoallergenic’, ‘partially hydrolysed’, and ‘extensively hydrolysed’.





