Chairperson: Marcus Maurer1
Speakers: Marcus Maurer,1 Miriam Wittmann2,3
1. Department of Dermatology and Allergy, Allergie-Centrum-Charité, Charité–Universitätsmedizin Berlin, Berlin, Germany
2. Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, Leeds, UK
3. NIHR Leeds Musculoskeletal Biomedical Research Unit, Chapel Allerton Hospital, Leeds, UK
Disclosure: Prof Maurer has been a speaker, advisory board member, and/or investigator for Adverum, Allakos, Alnylam, Amgen, Aralez, ArgenX, AstraZeneca, Attune, BioCryst, Blueprint Medicines, Centogene, Celldex Therapeutics, CSL Behring, Dyax, FAES, Genentech, GIInnovation, Jerini, Kalvista Pharmaceuticals, Kyowa Kirin, Eli Lilly and Company, Menarini, Novartis, LEO Pharma, Moxie, MSD, Pharming, Pharvaris, Roche, Sanofi/Regeneron, Shire, Takeda, Third HarmonicBio, UCB, and Uriach; received grants from Allakos, Amgen, AstraZeneca, BioCryst, Blueprint Medicines, Centogene, Celldex Therapeutics, CSL Behring, Dyax, FAES, Genentech, GIInnovation, Jerini, Kalvista Pharmaceuticals, Kyowa Kirin, Eli Lilly and Company, Menarini, Novartis, LEO Pharma, Moxie, MSD, Pharming, Roche, Sanofi/Regeneron, Shire, Takeda, UCB, and Uriach. Prof Wittmann has been a speaker, advisory board member, and/or investigator for UCB, Biogen, Novartis, Janssen, L’Oréal, AbbVie, and Celgene.
Acknowledgements: Writing assistance was provided by Dr Julia Granerod, London, UK, and Dr Allison Kirsop, Scientific Writers Ltd., Edinburgh, UK.
Support: The symposium and publication of this article was funded by Sanofi Genzyme and Regeneron.
Citation: EMJ Allergy Immunol. 2020;5[Suppl 1]:2-11.
Meeting Summary
Prof Maurer opened the symposium by noting its overall aim: to discuss advances in type 2 inflammatory diseases, specifically the shared pathophysiology and epidemiology of atopic dermatitis (AD) with other type 2 inflammatory diseases. AD is a type 2 inflammatory disease driven by the key and central cytokines IL-4 and IL-13, and this same underlying pathophysiology is shared in other dermatologic diseases, as well as diseases seen in other organ systems driven by type 2 inflammation. AD commonly coexists with other type 2 inflammatory diseases and the epidemiology is supported by studies which have revealed evidence of comorbid conditions. Therefore, systemic type 2 inflammation provides a unifying framework for a shared underlying pathophysiology and epidemiology in a broad range of diseases.
Prof Wittmann presented an overview of the clinical management and emerging treatments for AD and other type 2 inflammatory diseases. The first approved biologic for the treatment of AD, dupilumab, provides long-term efficacy with an acceptable safety profile in moderate-to-severe AD in adults and adolescents, and has significant therapeutic benefits in other type 2 inflammatory diseases. Emerging systemic therapies in AD include other biologics under investigation in Phase III trials as well as Janus kinase (JAK) inhibitors which have demonstrated improvement in signs and symptoms of AD over the short-term, but longer-term risk-benefit assessments are needed.
Shared Pathophysiology and Epidemiology of Atopic Dermatitis with Other Type 2 Inflammatory Diseases
Professor Marcus Maurer
Prof Maurer explored the common role of type 2 inflammation in the shared pathophysiology and epidemiology of AD and other type 2 inflammatory diseases. He explained how the immune system has evolved to respond to a variety of pathogens and how the primary immune cells and cytokines involved have been classified into three conceptual types of immunity: type 1, type 2, type 3.1 However, within this concept of immunity there is some overlap in the types of cells and mediators that cause inflammation. Type 1 immunity tends to target viruses, intracellular bacteria, and cancer cells. Examples of key type 1 immunity cells are type 1 innate lymphocytes [ILC1] and T helper type 1 [Th1]. Type 2 immunity primarily targets allergens and parasites. Key type 2 immunity cells and mediators are Th2 cells, type 2 ILC [ILC2], mast cells, T follicular helper cells [Tfh], basophils, and eosinophils; and key cytokines include IL-4, IL-5, IL-13, and IL-31. Type 3 immunity targets extracellular bacteria and fungi, with key cells including type 3 ILC [ILC3], Th17, and Th22.1 When dysregulated, these immune responses have the potential to cause inflammation which can result in disease.
Type 2 inflammation underlies many atopic disorders and significantly contributes to diseases such as AD, allergic rhinitis (AR), asthma, chronic rhinosinusitis with nasal polyps (CRSwNP), and eosinophilic esophagitis (EoE). Evolving from the concept of Th2 polarisation, Th2 cells are central mediators of the adaptive response in type 2 immunity.2 Cells in the innate immune response such as mast cells and basophils also produce IL-4, IL-5, and IL-13, and both Th2 and innate immune cells produce and respond to the key cytokines involved in type 2 inflammation.2,3 Atopic diseases are generally associated with IgE antibodies. Type 2 inflammation underlies allergic diseases with varying degrees of IgE-driven pathophysiology; AR, chronic spontaneous urticaria (CSU),4 and bullous pemphigoid (BP) are all generally associated with allergen/autoallergen-specific IgE. However, the clinical relevance of IgE in AD appears to be less significant. Approximately 50% of patients with severe asthma will have type 2 inflammation, which may also be accompanied by atopy,5 and severe CRSwNP is often associated with a type 2 inflammatory signature.6
Moreover, barrier disruption is common across multiple type 2 inflammatory diseases in different organ systems (e.g., asthma in the lungs, AD in the skin, EoE in the gut, and nasal polyps in the sinuses), contributing to further epithelial inflammation.
Type 2 Inflammation in Dermatology: A Focus on Atopic Dermatitis
Barrier disruption leads to epidermal alarmin production and initiation of type 2 immune responses.7 Continued activation of type 2 inflammation characterised by IL-4 and IL-13 at every disease stage maintains the inflammatory cycle, leading to further inflammation, itch, and skin barrier disruption.7,8 The presence of Th22, Th17, and Th1 cells is often characteristic of chronic disease, and highlights the complexity of processes taking place in the skin and other organs affected by type 2 inflammation. Thus, type 2 inflammation underlies the skin barrier dysfunction, inflammation, and itch in AD.9,10 The type 2 inflammatory cytokines that mediate chronic itch and inflammation in AD activate sensory neurons in the skin,11 priming immune cells to release proinflammatory and pruritogenic mediators.
Type 2 Inflammation in Dermatology: Other Dermatologic Diseases
In addition to AD, epidermal/dermal barrier disruption and pruritus are shared clinical features with other diseases affecting the skin, and these can be attributed, at least in part, to similar underlying type 2 inflammatory patterns and mechanisms. In CSU, patients present with pruritic wheals, angioedema, or both, and the underlying immunology reveals mast cell activation and IL-4-driven IgE-mediated autoimmunity.4,12 Type 2 inflammation also plays a role in chronic nodular prurigo (CNP), also known as prurigo nodularis, a chronic-relapsing skin condition characterised by intensely pruritic papulonodular lesions for which approximately one-half of all patients have a history of comorbid atopic diseases.13 Here, key type 2 cytokines, including IL-4 and IL-13, contribute to type 2 inflammation and may perpetuate the itch-scratch cycle causing further barrier disruption.7,11 In BP, a pruritic subepidermal blistering disorder, type 2 inflammation is again present and is thought to play a key role in IgE production,4 and is associated with the presence of eosinophil infiltration and circulating autoantibodies. Consequently, type 2 inflammation, including the key cytokines IL-4 and IL-13, may play a central role in all three diseases.
Diseases in Other Organ Systems
Moving on to discuss other organs affected by the same type 2 inflammation patterns and mechanisms, Prof Maurer emphasised the level to which these different elements of type 2 inflammation are shared. In the context of asthma (lower) and CRSwNP (upper) inflammatory airway diseases, type 2 inflammation underlies many clinical features observed in these diseases. Although complex, shared features include the initial barrier disruption through damage to the epithelial mucosa, which results in the activation of alarmins. In both of these airway diseases, IL-4, IL-5, and IL-13 contribute to the presence of eosinophils in many patients. B-cell class switching and the production of IgE is also observed, as well as M2 macrophage polarisation.8,14 In EoE, a type 2 inflammatory disease found in the oesophagus, type 2 inflammation initiates mast cell-driven recruitment of eosinophils through type 2 cytokines, where IL-5, IL-4, and IL-13 are again important drivers of the influx of inflammatory cells also seen in the skin and airways. IgE, directed by type 2 cytokines, also plays a role in this disease.8,15 Across these diseases seen in different organs such as the skin, airways, or gut, epithelial alarmins, type 2 cytokines, infiltrating cells, and sensory neuron involvement are common features.
Epidemiology of Type 2 Inflammatory Diseases
As discussed, the epidemiology of type 2 inflammatory diseases suggests that patients with one type 2 inflammatory disease have an increased risk of developing another.13,16-21 A study revealed that in patients with AD, asthma occurs at a higher rate than in the general population, in up to 34% of patients with severe AD.21 AD is slightly increased in patients with CSU,20 and an atopic predisposition was observed in almost one-half of patients with CNP.13 Data from the Danish nationwide health registry provided evidence for an association between AD and the risk of developing other dermatologic comorbidities, showing an increase in the prevalence of chronic urticaria in patients with AD.22 This association reveals how underlying mechanisms not only drive a disease with a type 2 inflammatory signature, but also increase the probability of coexisting type 2 inflammatory diseases in the same patient. Indeed, it is thought that the prevalence of AD in patients with severe asthma or other comorbid type 2 inflammatory disease may be underestimated, supported by data from registry studies and clinical trials which emphasise the increased risk of comorbid type 2 inflammatory disease (Figure 1).23 In the airways, the coexistence of AR and asthma is well documented.16-19 A recent study showed that 38% of patients with severe asthma had concomitant AD, an increase on previous data.24 Two Phase III studies of dupilumab in patients with moderate-to-severe asthma revealed that 10% of patients had baseline comorbid AD.25,26
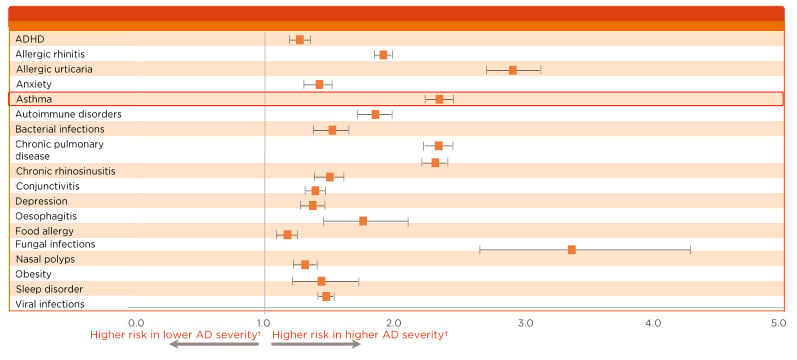
Figure 1: Data highlighting the burden of atopic dermatitis in the USA relative to the general population.
Increased severity of atopic dermatitis is associated with an increased risk for comorbid asthma and other type 2 inflammatory diseases.
*p<0.0001 for all aOR.
†AD patients were stratified by disease severity (higher, lower) using treatment as a surrogate measure of severity.
Analysis of Healthcare Claims Data from adult AD patients in the Commercial (n=83,106), Medicare (n=31,060), and Medi-Cal (n=5550) databases. Data from adult AD patients were matched 1:1 to non-AD controls by demographic characteristics. Analysis of burden of AD (‘severity’) encompassed comorbidities, healthcare resource utilisation, and costs.
AD: atopic dermatitis; ADHD: attention deficit hyperactivity disorder; aOR: adjusted odd ratio.
Adapted from Shrestha et al.23
Data from the BioDay Registry also revealed high rates of AD, AR, asthma, and food allergy in patients who had at least one other type 2 inflammatory disease,27,28 and data from Phase III clinical trials of dupilumab further supported this finding. The high comorbidity rate within type 2 inflammatory diseases may be underappreciated in clinical practice.
Evidence from Dupilumab Clinical Trials
In the CHRONOS (N=740)29 and CAFÉ (N=325)30 Phase III clinical trials, patients with moderate-severe AD were found to have AR (43% and 57%, respectively), asthma (39% and 41%, respectively), and food allergies (33% and 45%, respectively). In the QUEST (N=1,902)25 and VENTURE (N=210)26 Phase III clinical trials, patients with moderate-severe asthma were found to have AR (68.6% and 57.0% , respectively), and AD (10.3% and 10.0%, respectively). In the SINUS 24 and SINUS 52 Phase III clinical trials,31 59% of patients with CRSwNP in the intention-to-treat overall population also had asthma, and 80% of patients had a previous medical history for other type 2 inflammatory diseases.
Heterogenous Developmental Profiles of Type 2 Inflammatory Diseases
Results of a meta-analysis show that approximately one-half of all patients with AD may have an atopic family history,32 and parental history of AD confers the highest risk for children to develop the disease.33 Such information is, therefore, important for management of patients with AD and other type 2 inflammatory diseases. The ‘atopic march’ concept is evolving from a specific sequence of occurring atopic diseases to an accumulation of risk factors leading to the increased likelihood of multiple atopic comorbidities in the same patients. In paediatric patients, the risk of disease progression and for developing atopic comorbidities increases with the accumulation of risk factors; this most often occurs with very early age of onset (3 months to 2 years), family history of atopy in one or both parents, moderate-to-severe disease, polysensitisation, living in an urban environment, and the presence of filaggrin mutation. Referral for specialised treatment is recommended for patients with these risk factors.34
In summary, AD is a type 2 inflammatory disease driven by the key and central cytokines IL-4 and IL-13.35 Elements of type 2 inflammation are also present in other dermatologic diseases such as CSU, CNP, and BP.4,36 Beyond dermatology, diseases in other organ systems share key features of the same underlying pathophysiology (e.g., the respiratory and gastrointestinal tracts), and importantly, this shared pathophysiology is responsible for the coexistence of more than one type 2 inflammatory disease in many atopic patients.8 The epidemiology of type 2 inflammatory diseases is supported by clinical studies which provide evidence of comorbid conditions in patients with another type 2 inflammatory disease,25,26,29-31 and recognition of these coexisting systemic diseases may influence patient management.
Clinical Management of Atopic Dermatitis and Other Type 2 Inflammatory Diseases
Professor Miriam Wittmann
Prof Wittmann presented an overview of the clinical management and emerging treatments in AD. The concept of a shared pathophysiology and the importance of IL-4, IL-5, and IL-13 with other mediators and cells that have a key role in the type 2 inflammatory response can be applied to the development of novel treatments for type 2 inflammatory diseases. Exploring the current portfolio of approved targeted therapies, and those under development, Prof Wittmann provided a detailed look at the treatment approaches for AD and other type 2 inflammatory diseases.
Targeted Biologic Therapies
For the treatment of moderate-severe AD, dupilumab is the only approved systemic biologic therapy. It targets type 2 inflammation, and acts by blocking both IL-4 and IL-13 signalling by binding to the the anti-IL-4 receptor α (anti-IL-4Rα). In later development stages, potential new treatments for AD include another three biologics: tralokinumab and lebrikizumab are antibodies targeting IL-13, while nemolizumab targets IL-31Rα and prevents the signalling of IL-31. In addition, there are small molecules in later phases of clinical investigation for the systemic treatment of AD: the JAK inhibitors abrocitinab and upadacitinab (JAK1 inhibitors), and baricitinib (JAK1/2 inhibitor). Other type 2 inflammatory diseases are already treated using targeted therapies; in asthma, dupilumab (targeting IL-4 and IL-13 signalling), omalizumab (anti-IgE), and others which target IL-5 signalling (mepolizumab, reslizumab, and benralizumab) are all approved systemic therapies, while dupilumab is also approved for use in CRSwNP. There are a range of different biologics at various stages of development which target distinct phases of AD pathogenesis.
Clinical Effects of Dupilumab
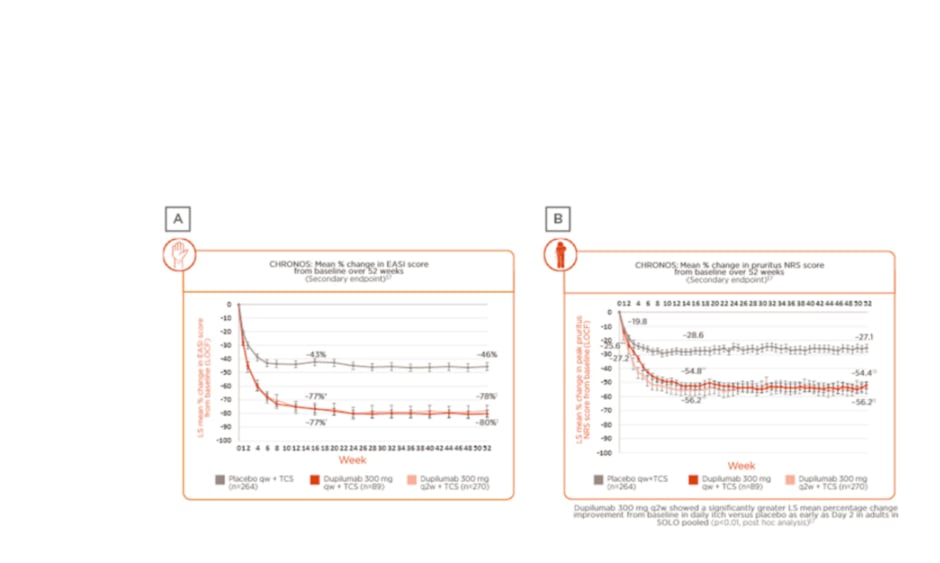
Prof Wittmann presented data from the 52-week, Phase III, randomised, placebo-controlled trial LIBERTY AD CHRONOS, which studied adults with moderate-to-severe AD inadequately controlled by topical treatment.29 In this study, patients were randomly assigned (3:1:3) to subcutaneous dupilumab 300 mg once a week (qw), 300 mg every 2 weeks (q2w), or placebo. In the overall study population of 623 patients assessed over 52 weeks, data from the trial showed long-term sustained clinical efficacy, as determined by the mean percentage change from baseline in both Eczema Area and Severity Index (EASI) score (Figure 2A)29,37 and pruritus numerical rating score (NRS) (Figure 2B).29,37 Results revealed that the observed clinical effect on the skin appeared within the first few weeks of treatment, peaking at approximately 16 weeks (a case study patient was included in the presentation to demonstrate treatment outcomes at Week 16), and maintained over 52 weeks.
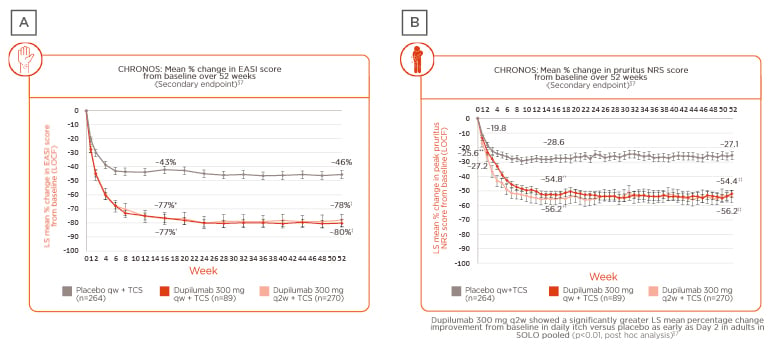
Figure 2: Change in skin and itch scores in patients enrolled in the LIBERTY AD CHRONOS clinical trial.
A) Weekly EASI score: mean percentage change from baseline over 52 weeks; B) weekly pruritus NRS score: mean percentage change from baseline over 52 weeks.29,37
*p<0.0001, dup q2w plus TCS versus placebo plus TCS (FAS).
†p<0.0001, dup qw plus TCS versus placebo plus TCS (FAS).
‡p<0.0001, dup q2w plus TCS versus placebo plus TCS and dupilumab qw plus TCS versus placebo TCS (FAS-52).
**p=0.0077, dup qw plus TCS versus placebo plus TCS (FAS).
††p<0.0001, dup q2w plus TCS and dup qw plus TCS versus placebo qw plus TCS (FAS).
‡‡p<0.0001, dup q2w plus TCS and dup qw plus TCS versus placebo qw plus TCS (FAS-52).
§Dose regimen in the ongoing Phase III, multicentre, open-label extension trial in adults with moderate-to-severe AD was 300 mg qw.
FAS-52: patients in the full analysis set who had completed 52 weeks of treatment and were evaluated for efficacy outcomes by the cut-off date for U.S. Food and Drug Administration (FDA) submission.
dup: dupilumab; EASI: Eczema Area and Severity Index; FAS: full analysis set; LOCF: last observation carried forward; LS: least squares; NRS: numerical rating score; q2w: every 2 weeks; qw: weekly; TCS: topical corticosteroids.
Adapted from Blauvelt et al.29 and Silverberg et al.37
Post hoc analysis showed that improvement in itch severity occurred as quickly as 2 days after treatment initiation in the intervention arm versus placebo (p<0.01), and again, clinical benefit was maintained over 52 weeks.37
A follow-up open-label extension (LIBERTY AD OLE) study of dupilumab 300 mg as a weekly dose in adults with moderate-to-severe AD demonstrated long-term safety as well as sustained, clinical efficacy up to 3 years.38 The significant improvement of both EASI and NRS scores was maintained over the 3-year period. As for placebo-controlled trials, higher rates of conjunctivitis and injections site reaction were observed with dupilumab compared to placebo. Conjunctivitis was observed in <20% of patients in both the LIBERTY AD CHRONOS 52-week trial (rate for conjunctivitis in the placebo plus TCS group in the CHRONOS trial was 8%) and the LIBERTY AD OLE 3-year follow-up study, suggesting no increase in the number of cases or worsening of the condition over 3 years. Injection site reactions were considered mild and did not lead to discontinuation of therapy. A concern at this time is how patients can be treated while we are experiencing the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; coronavirus disease 2019 [COVID-19]) pandemic. In clinical trials, dupilumab was associated with reduced risk of serious/severe infections and non-herpetic skin infections and did not increase overall infection rates versus placebo in patients with moderate-to-severe AD.39 In a limited number of AD patients treated with dupilumab, no deterioration of symptoms related to COVID-19 or increased risk of acquiring COVID-19 was observed (case study with 2 patients).40
Dupilumab in Adolescents
Similar data are available on the efficacy of dupilumab monotherapy in adolescents with moderate-to-severe AD (dupilumab 300 mg q4w, [n=84]; dupilumab 200/300 mg q2w, [n=82]; placebo, [n=85]). Long-term sustained clinical efficacy comparable with the study in adults was observed over 16 weeks, and maintained over 52 weeks.41,42 The dupilumab 300 mg q2w regimen group showed a significantly greater mean percentage change improvement from baseline in daily itch severity compared with the placebo group, and this change occurred as early as Day 5 after treatment initiation (p<0.05, post hoc analysis).37 The long-term safety data over 52 weeks was consistent with that seen in adults. There were also less infections observed in dupilumab-treated patients than placebo-treated patients, similar to observations in adult patients. Conjunctivitis was observed in up to 10.8% of patients in the treatment arms versus 4.7% in the placebo group.41,42
Biologics Under Investigation in Atopic Dermatitis
Tralokinumab
Data from a Phase IIb43 study of tralokinumab, which targets the type 2 cytokine IL-13, showed clinical efficacy as measured by improvement of EASI (coprimary endpoint) and pruritus NRS (secondary endpoint) scores from baseline. EASI score was significantly different in both 150 mg and 300 mg intervention arms versus placebo group: -4.4% (n=51; p=0.03) and -4.9% (n=52; p=0.01), respectively. Improvement in itch severity was observed at Week 12 for all three intervention arms (45, 150, and 300 mg doses). There was no significant signal for infection and no safety concerns over the 12-week treatment period.
Lebrikizumab
Data from a Phase IIb study44 of lebrikizumab, also an anti-IL-13 therapy, showed clinical efficacy as measured by both EASI and pruritus NRS scores at Week 16. Compared with the placebo group (EASI mean percentage change of 41.1% from baseline, [n=52]), there were dose-dependent statistically significant differences in EASI score for each intervention group: 125 mg q4w (62.3%, n=73, p=0.02); 250 mg q4w (69.2%, n=80, p=0.002), and 250 mg q2w (72.1%, n=75, p<0.001). Improvement in itch severity was observed by Day 2 for the high-dose intervention group and the safety profile over the 16-week period was comparable with that of dupilumab and tralokinumab. Conjunctivitis and injection site reaction signals were observed, and there was no suggestion of increased infection.
Nemolizumab
Nemolizumab targets the type 2 cytokine IL-31, identified in the pathophysiology of AD and other skin diseases as the cytokine mainly involved in pruritus. Data from a Phase IIb trial45 with intervention arms of 10 mg, 30 mg, and 90 mg nemolizumab versus placebo showed a difference in mean percentage change in EASI score from baseline of 16.7% for the 30 mg dose at 24 weeks, compared with placebo. The clinical effect observed in the mean percentage change in pruritus NRS score from baseline over 24 weeks was more pronounced. All three doses of nemolizumab were associated with a rapid decrease in pruritus NRS scores compared with placebo, with statistically significant differences starting as early as Week 1. The adverse event profile of this biologic therapy is slightly different from the others discussed and included the possibility of increased infection risk as well as an increase in asthma events.
JAK inhibitors Under Investigation in Atopic Dermatitis
The JAK family is a small group of signalling molecules which are central to most cytokine receptor signalling cascades, including type 2 and type 1 cytokine receptors. These molecules inhibit the activity of one or more JAK enzymes (JAK1, JAK2, JAK3, TYK2) and as a result have a broad range of action.46 Their use in other conditions such as rheumatoid arthritis has revealed good efficacy potential against inflammatory diseases, but also some safety concerns.
Baricitinib
Baricitinib is a JAK 1/2 inhibitor with data available from the Phase III BREEZE-AD7 trial47 which evaluated two dosing arms (2 mg once daily plus TCS, [n=109] and 4 mg once daily plus TCS, [n=111]) and placebo plus TCS (n=109). Over 16 weeks, there was significant efficacy measured by EASI and pruritus NRS scores for both dosing arms (p≤0.05 and p≤0.01, respectively) compared with placebo. There were also statistically significant improvements in mean percentage change from baseline in pruritus NRS score versus placebo (p≤0.01). The safety overview during the 16-week treatment period showed changes in blood creatine phosphokinase for the higher dose group which may indicate a monitoring need. A number of patients also reported headache and nausea. Long-term data beyond 16 weeks will help to accurately assess the risk of infection associated with this JAK 1/2 inhibitor.
Abrocitinib
Data from the JADE-MONO-2 Phase II study46,48 showed a significant clinical effect in the number of patients achieving 75% improvement in EASI score from baseline (EASI 75) over 12 weeks for both treatment arms (100 mg, [n=158]; and 200 mg, [n=155]; p≤0.0001) versus placebo (n=78). As early as Day 2, there were significant differences in peak pruritus NRS scores for both treatment arms (p<0.05) versus placebo, with further improvement over 12 weeks (p≤0.0001). The safety profile over the 12-week treatment period suggested an increased risk of headache, nausea, vomiting, and acne in ≥5% of patients in both treatment arms.49
Upadacitinib
Phase IIb efficacy data50 revealed statistically significant differences in the mean percentage change of EASI score from baseline over the 16-week endpoint for all treatment arms (7.5 mg, [n=42], p≤0.05; 15 mg, [n=42], p≤0.001; and 30 mg, [n=42], p<0.001) versus placebo (n=41). All three groups showed a comparable improvement in the mean percentage change from baseline in pruritus NRS score versus placebo, effective from Week 1. The safety profile over the 16-week treatment period showed changes in blood creatine phosphokinase, acne was present in up to 14% of patients, and, long-term data are needed to accurately assess the risk of infection beyond 16 weeks.
Beyond Atopic Dermatitis: Approved Biologic Therapies Targeting Type 2 Inflammation in Other Diseases
Systemic treatments for other type 2 inflammatory diseases include a similar range of approved and investigatory biologic therapies, as discussed for AD. Dupilumab (anti-IL-4α), mepolizumab, reslizumab, and benralizumab which target IL-5, and omalizumab which targets IgE, have all been approved for use in asthma. In AD, trials for IL-5 targeting biologics were halted due to poor efficacy outcomes. In asthma, trials for IL-13 targeting biologics (tralokinumab and lebrikizumab) were halted, whereas in AD, these biologics showed efficacy in Phase IIb trials.
Summary
The clinical experience with novel drug categories such as biologics and JAK inhibitors continues to inform our understanding of the pathophysiology of type 2 inflammatory diseases. Evidence of type 2 inflammation as a shared pathophysiological feature is emerging for a broad range of skin diseases (AD, CSU, CNP, and BP), as well as diseases involving other organs such as asthma, CRSwNP, and EoE. Moreover, the overlapping epidemiology of type 2 inflammatory diseases provides additional evidence of a shared underlying disease pathway. Targeting type 2 inflammation present in organ-specific diseases has allowed patients with historically difficult-to-control diseases to receive effective treatment with less impact on other elements of the immune system.
One approved biologic, dupilumab, has demonstrated efficacy in designated patient populations with different organ-specific type 2 inflammatory diseases: moderate-to-severe AD, moderate-severe asthma, and CRSwNP. As the first approved targeted systemic therapy in moderate-severe AD, dupilumab treatment results in sustained long-term safety and efficacy in adults for up to 3 years. Furthermore, the investigational biologics targeting AD tralokinumab, lebrikizumab, and nemolizumab have all met their clinical efficacy endpoints. Clinical evaluations of small molecules JAK inhibitors in patients with AD suggest that these drugs may be safe and efficacious in the short term, with longer-term data not yet available.
In addition to dupilumab, the biologics omalizumab, mepolizumab, reslizumab, and benralizumab are all approved for use for severe asthma with type 2 inflammation, and several Phase II/III trials with these biologics are either completed or underway in AD, AR, CRSwNP, and EoE. Thus, for the first time in decades, thanks to a better understanding of the pathophysiology of type 2 inflammatory diseases, novel therapeutic options are available or will be available in the near future to better target type 2 inflammation and more effectively treat chronic diseases such as AD, asthma, CRSwNP, and EoE.
MAT-GLB-2001143 July 2020