Meeting Summary
This symposium, held on the first day of the 2024 European Academy of Allergy and Clinical Immunology (EAACI) Congress, aimed to broaden clinicians’ understanding of indolent systemic mastocytosis (ISM) diagnosis, the tools available to help assess the disease burden and severity of symptoms, and the use of symptomatic therapies and novel precision medicines.
Speakers described the hallmark symptoms of ISM as typical skin lesions, anaphylaxis and other mast cell-mediator release symptoms, and bone mass loss. Serum tryptase testing and screening for KIT D816V mutation using high-sensitivity PCR assays were considered key elements of ISM diagnosis, though bone marrow study becomes essential for diagnostic confirmation.
A stepwise approach to symptomatic management was recommended, with the caveat that symptomatic treatments are used off-label for ISM. It was noted that polypharmacy is often required to achieve adequate responses, and that novel therapeutics are needed in patients with inadequately controlled disease, highlighting the remaining unmet need.
Speakers stressed that the success of ISM therapy should be measured primarily by symptom improvement, and that validated tools are needed to assess a broad range of patient-reported symptoms and quality of life. Several current and upcoming tools for this purpose were described.
Clinical trial data of several precision medicines for ISM were described. These included avapritinib, recently authorised by the European Medicines Agency (EMA) for use in adults with ISM with moderate-to-severe symptoms inadequately controlled on symptomatic treatment, and investigational medicines bezuclastinib and elenestinib.
The overall message of the symposium was that, in this era of precision medicine, the emergence of novel targeted treatments brings an opportunity to transform the management of ISM.
INTRODUCTION
Systemic mastocytosis (SM) is a rare myeloid neoplasm characterised by clonal proliferation and accumulation of neoplastic mast cells in various organs.1,2 Activated mast cells release pro-inflammatory mediators, which can result in severe and often unpredictable symptoms, including life-threatening anaphylaxis.2 The indolent form (ISM) is the most common subtype of SM, representing approximately 90% of SM cases.1
Massimo Triggiani, Professor of Internal Medicine at the Division of Allergy and Clinical Immunology, University of Salerno, Italy, explained that mast cells are unique among blood cells in expressing high levels of the KIT receptor at all stages of maturation.3 In the vast majority (approximately 95%) of SM cases, the primary driver of disease is KIT D816V mutation, which results in increased proliferation and activation of mast cells,1 and this provides an opportunity for targeted treatments.
Over the past few years, major advances have been made in developing precision medicine approaches for ISM, with the emergence of KIT-targeted tyrosine kinase inhibitors (TKI).4-7 To make the best use of these opportunities, Triggiani stressed that a precision medicine approach to therapy needs to begin with accurate diagnosis and the use of patient-reported outcome measures to assess individual patient experience and disease burden.8
PRECISION MEDICINE BEGINS WITH ACCURATE DIAGNOSIS: HALLMARK SYMPTOMS AND DIAGNOSTIC PARAMETERS FOR INDOLENT SYSTEMIC MASTOCYTOSIS
Because mast cells have multiple activating receptors, and release a wide range of proinflammatory and vasoactive mediators,9 patients with ISM can experience a broad spectrum of signs and symptoms (Figure 1).10
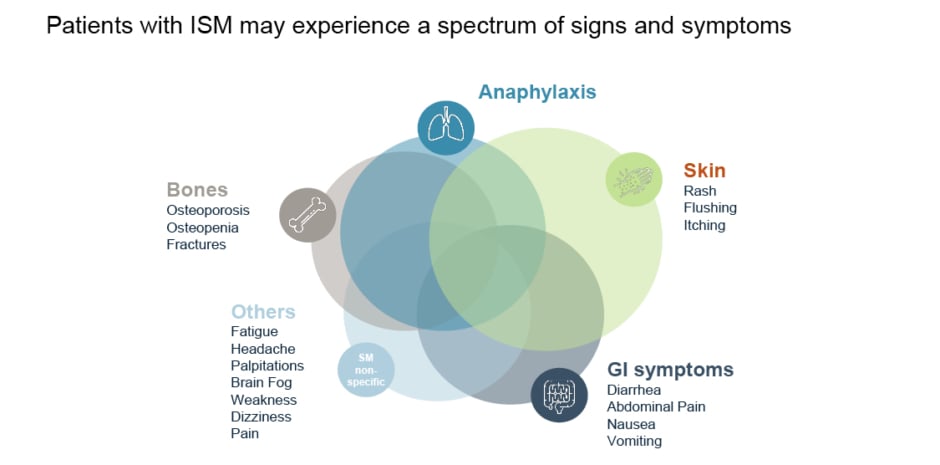
Figure 1: Clinical signs and symptoms associated with indolent systemic mastocytosis.10
GI: gastrointestinal; ISM: indolent systemic mastocytosis.
Iván Álvarez-Twose, Haematologist at the Institute of Mastocytosis Studies of Castilla-La Mancha (CLMast), Virgen del Valle Hospital, Toledo, Spain, explained that ISM is a highly heterogenous subtype of SM, and this can present a challenge for timely diagnosis. Indeed, the average time from initial symptoms to diagnosis is 6 years.11
Despite the wide range of signs and symptoms, Álvarez-Twose emphasised several hallmark symptoms that are often associated with ISM.
Skin Lesions
These are the most common signs of SM, observed in over 80% of patients.12 Patients with ISM commonly present with typical, small, monomorphic, maculopapular, brown or red skin lesions (mastocytosis in the skin [MIS]).12 The majority of adult patients with MIS will have a systemic involvement, e.g., SM with mast cells present not only in the skin but also in other organs such as the bone marrow (BM), gastrointestinal tract, spleen, or liver.2,12 As a general rule, all adult patients with MIS should undergo a BM aspirate and biopsy to confirm the ISM diagnosis.12 The Fuchs risk score proposed by the European Competence Network on Mastocytosis (ECNM) can be used to predict SM in patients with MIS.13 It includes serum tryptase levels, bone symptoms or osteoporosis, and constitutional or cardiovascular symptoms, with a score >2 being associated with high probability of SM.13
Anaphylaxis and Other Mast Cell-Mediator Release Symptoms
Other patients may present without skin lesions but with different symptoms related to the release of mast cell mediators.13 Mast cell activation (MCA) symptoms are normally acute and systemic, with involvement of ≥2 organ systems, such as the skin, gastrointestinal tract, respiratory system, or cardiovascular system.13-15 MCA can cause a broad spectrum of symptoms, such as diarrhoea, vomiting, flushing, pruritus, hypotension, rhinorrhoea, and shortness of breath, among other manifestations.15 Anaphylaxis is also common (up to half of adult patients) and often severe (approximately 48% of reactions) in SM.14,15 In patients with MCA symptoms but without MIS, the REMA (Red Española de Mastocitosis [Spanish Mastocytosis Network]) score can be used to predict SM with high sensitivity and specificity.16 The REMA score considers sex, serum tryptase levels, pruritis, hives/angioedema, and presyncope/syncope.16
Bone Mass Loss
Some patients with ISM can present with bone fractures due to osteoporosis.6,17 Osteoporosis is quite common in patients with ISM, it can occur in one-third of patients during the course of the disease, and its prevalence may be underestimated.17 Overall, ISM is diagnosed in 0.5% of patients with osteoporosis, but ISM incidence is 10 times higher in young men with osteoporosis (>5%) and should raise high suspicion of SM in this population.18
KIT D816V mutation leads to constitutive activation of the KIT receptor, and abnormal proliferation and accumulation of mutated mast cells in various organs.3,8 Activated mast cells release various mediators, including tryptase, a highly specific marker for mast cells.8,19 These two biomarkers, KIT D816V mutation and serum tryptase, can be measured in peripheral blood and serve as key elements of SM diagnosis.9 The high-sensitivity PCR assays (e.g., droplet digital PCR or allele-specific oligonucleotide PCR) are extremely useful for the detection of KIT D816V mutation in peripheral blood from patients with SM. Next-generation sequencing should not be used for screening of the KIT D816V mutation due to lower sensitivity.20,21 Finally, a complete BM study should be performed to confirm the diagnosis of SM.12
Álvarez-Twose highlighted the value of flow cytometry for the diagnosis of SM, as it can be useful for the identification, enumeration, and characterisation of neoplastic BM mast cells even when they coexist with normal BM mast cells.22
Diagnosis of SM requires one major and one or more minor, or at least three minor criteria from the following:23
Major Criteria
- Multifocal dense infiltrates of mast cells (≥15 mast cells in aggregates) in BM biopsies or extracutaneous organs
Minor Criteria
- ≥25% atypical or spindle-shaped mast cells
- KIT-activating KIT gene mutation (e.g., D816V) in BM biopsies or extracutaneous organs
- Aberrant CD2, CD25, and/or CD30 expression on mast cells
- Baseline tryptase ≥20 ng/mL
Algorithms for the diagnosis of SM are available in the literature and can help to sub-classify SM into six subtypes of the disease, including ISM.9,24 Moreover, Álvarez-Twose shared that the GEMAST app (Euromedice, Educiones Médicas SL Badalona, Spain) has been developed and can be downloaded free of charge, which can help in the identification, diagnosis, classification, and prognostic stratification of patients with SM.
DISEASE BURDEN IN ISM: USING PRO TOOLS TO ASSESS THE INDIVIDUAL PATIENT EXPERIENCE
Frank Siebenhaar, Assistant Professor at the Institute of Allergology, Charité – Universitätsmedizin Berlin, Germany, stressed that around 40% of patients with ISM experience moderate-to-severe mastocytosis-related symptoms, and a similar proportion experience a moderate-to-severe quality of life (QoL) impairment, representing a substantial unmet need.25,26
This has a negative impact on a patient’s ability to work; one-third (32%) of patients with non-advanced SM need to reduce their working hours, 14% reported having been on medical disability, and 12% reported that they do not work at all due to their disease (N=98).27 This impact occurs despite patients taking multiple medications to control their symptoms; approximately two-thirds (75%) of patients with ISM have taken ≥4 classes of drugs to treat their disease, representing a substantial polypharmacy burden (Blueprint Medicines Corporation, data on file [REF-00686]).
Siebenhaar explained that there are several patient-reported outcome measure tools that can be used to assess disease burden in ISM, covering areas such as symptom burden, QoL impairment, and disease control. These tools can enable clinicians and patients to monitor the disease.
Symptom burden can be assessed using the Mastocytosis Activity Score (MAS), the Mastocytosis Symptom Assessment Form, or the Indolent Systemic Mastocytosis Symptom Assessment Form (ISM-SAF). The MAS assesses nine items across three domains: skin (itching, wheals, and flushing symptoms); gastrointestinal tract (diarrhoea and abdominal pain); and “other” (muscle/joint pain, fatigue, headache, and concentration). Each item scores from 0–4 points by severity, and total points are normalised to a maximum total score of 100.
QoL impairment can be assessed using one of the Mastocytosis Quality of Life questionnaires: MC-QoL or MQLQ. The MC-QoL questionnaire assesses 27 items across four domains: symptoms, functioning/social life, emotions, and skin. Each item scores from 0–4 points by severity, and total points are normalised to a maximum total score of 100.
Another tool has recently been developed to assess disease control in adults with cutaneous mastocytosis or ISM: the Mastocytosis Control Test (MCT; manuscript in preparation). This is a short simple test which assesses five items, each of which can score from 0–4 points. The maximum score is 20 points, and a score of ≥13 reflects a well-controlled disease.
Each of these tools (MAS, MC-QoL, MCT) are available in several different languages, free-of-charge for routine clinical use and non-commercial research.28 The MASTHAVE Mastocytosis App is also being developed as a collaboration between the Global Allergy and Asthma Excellence Network (GA2LEN), MOXIE, and Blueprint Medicines, which will include many of these tools and allow patients to track their SM disease burden in a digital app and share their data with their clinician.
SYMPTOMATIC MANAGEMENT OF ISM: ARE WE DOING ENOUGH?
In SM, greater disease burden (aggressiveness) is associated with more prominent organopathy, whereas lesser disease burden is associated with more prominent mast cell mediator symptoms (such as skin, gastrointestinal, cardiovascular, and neurologic symptoms).29 Consequently, symptoms of mast cell mediator release have been observed in 91% of patients with ISM (N=76).30
Common presenting symptoms of MCA include flushing and pruritis of the skin, dyspnoea and wheezing in the respiratory system, abdominal cramps and diarrhoea in the gastrointestinal system, and hypotension and syncope in the cardiovascular system, as well as anxiety and cognitive impairment.31 In severe cases, anaphylaxis can occur, and this condition has been observed in 22–49% of adults with mastocytosis.31 Anaphylaxis in the general adult population is most likely to be triggered by food or medication, whereas in patients with SM, Hymenoptera sting (e.g., bees, wasps, or ants) is the most common trigger.32
Álvarez-Twose explained that a stepwise approach to symptomatic management is generally used for ISM in clinical practice, though he stressed that all symptomatic treatments are used off-label.
The basic management for ISM involves prophylactic avoidance of known triggers, and therapy with antihistamines to block the H1 and H2 receptors.9
Additional therapies and measures may be required, such as proton-pump inhibitors or mast-cell stabilisers to manage refractory gastrointestinal symptoms; mast-cell stabilisers, aspirin, or anti-leukotriene to control refractory flushing, tachycardia, or hypotension; UV-light therapy for refractory skin symptoms; or corticosteroids to manage other refractory symptoms.9 In some patients with ISM who experience refractory symptoms, additional specific therapies may be appropriate, such as adrenaline (for anaphylaxis), anti-IgE therapy, and TKIs.9
The adjuvant approaches in ISM include calcium/vitamin D supplements, bisphosphonates, or denosumab to manage osteoporosis; life-long venom immunotherapy to control Hymenoptera venom allergy; and anxiolytics, antidepressants, or psychotherapy to address stress-induced anaphylaxis, depression, or anxiety.33
Overall, a combination of symptomatic treatments rather than a single agent may be needed to achieve adequate control of symptoms.9 There remains an unmet need for patients with inadequately controlled disease despite the use of multiple drugs.2
PRECISION MEDICINE ERA IN ISM: CURRENT AND EMERGING TARGETED TREATMENTS
Over the last few years, several novel precision medicines have been (or are being) developed to target human mast cells.34 These include inhibitors of mast-cell mediators or their secretion, such as lirentelimab (a siglec-8 inhibitor), and ibrutinib (a Bruton’s tyrosine kinase [BTK] inhibitor), and agents that deplete mast-cell numbers, such as barzolvolimab (a KIT antibody) and the TKIs that target the KIT receptor: avapritinib, bezuclastinib, elenestinib, masitinib, and midostaurin.35
Siebenhaar stressed that symptom improvement is the gold standard by which the success of ISM therapy should be measured, since clinical studies have shown that tryptase levels do not correlate with symptom severity at baseline.6 Therefore, validated tools are needed to assess response to therapy and symptom improvement across a broad range of symptoms.
Avapritinib*
*Avapritinib is authorised by the EMA for use in adult patients with ISM with moderate-to-severe symptoms inadequately controlled on symptomatic treatment (ava EMA SmPC). This medicinal product is subject to additional monitoring.
Avapritinib is a tyrosine kinase inhibitor designed to selectively target the KIT D8161V mutation, the main driver of SM disease.7 It was authorised by the EMA for use in ISM based on results from the PIONEER clinical study (NCT03731260),36 a randomised, double-blind, placebo-controlled trial of avapritinib in adult patients with ISM.7
In PIONEER, the first randomised, double-blind, placebo-controlled Phase II trial, patients with moderate-to-severe ISM (ISM-SAF total symptom score [TSS] >28) were randomised, 2:1 to 25 mg avapritinib once-daily (n=141) or placebo (n=71), both with best supportive care (BSC).4 The primary endpoint was the mean change in TSS (range 0–110) from baseline to Week 24. Key secondary endpoints included safety, the proportion of participants achieving ≥30% and ≥50% TSS reduction, and the proportion of participants achieving >50% reduction in serum tryptase, BM mast cell burden, and blood KIT D816V variant allele fraction (VAF).4 The 5-year open-label extension aims to collect data on long-term efficacy and safety.4
Overall, avapritinib significantly improved ISM symptoms and underlying mast cell burden versus placebo, indicative of disease modification.4 The primary endpoint was met for this trial.4 At Week 24, the mean decrease from baseline in TSS was 15.6 points (95% CI: -18.6 to -12.6) with avapritinib versus 9.2 points (95% CI: -13.1 to -5.2) with placebo (p=0.003).4
Symptom reduction was observed across all domains of the ISM-SAF with avapritinib versus placebo, including abdominal pain, nausea, diarrhoea, skin spots, itching, flushing, fatigue, bone pain, brain fog, headache, and dizziness (Figure 2),37 and symptom improvement with avapritinib was maintained up to Week 48.4 After 24 weeks, patients treated with placebo switched to avapritinib and achieved similar improvement in symptoms at Week 48.4
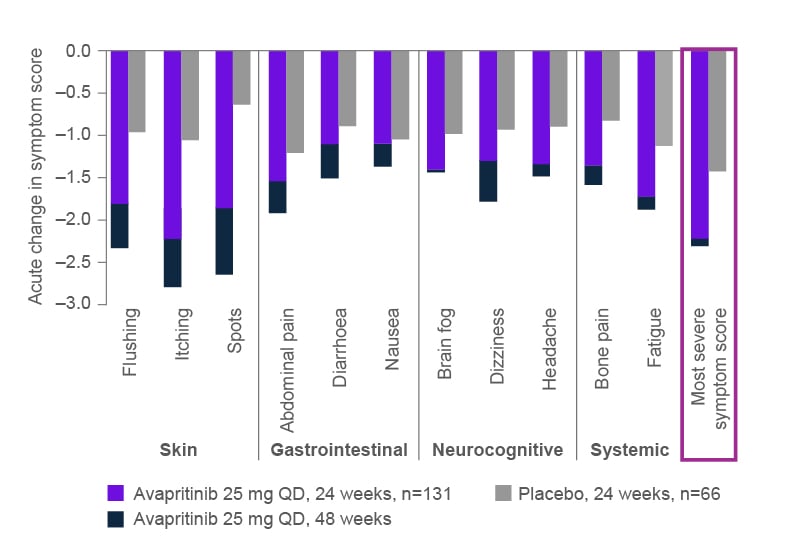
Figure 2: Mean total symptom score absolute change from baseline to 24 weeks, individual Indolent Systemic Mastocytosis Symptom Assessment Form, by treatment group.37
TSM: total symptom score; QD: once daily.
Avapritinib also showed reductions in all biomarkers of mast cell burden. Over 24 weeks of treatment, 54% of patients treated with avapritinib versus none (p<0.001) in the placebo group achieved >50% reductions in serum tryptase, 53% of patients treated with avapritinib versus 23% (p<0.001) in the placebo group achieved >50% reductions in BM mast cell burden aggregates, and 68% of patients treated with avapritinib versus 6% (p<0.001) of the placebo group achieved >50% reductions in KIT D816V VAF.4 Importantly, at baseline, 62% of patients in the avapritinib group used ≥3 symptomatic treatments, and after 48 weeks of treatment, one-third of patients had decreased their BSC use and 4% were able to completely discontinue BSC, contributing to reduction in polypharmacy.39 During this same period, the QoL of patients treated with avapritinib also improved, with a decrease in the mean MC-QoL total score of 34% (95% CI: -40% to -29%) with avapritinib versus 18% (95% CI: -25% to -11%) with placebo.4 The changes in MC-QoL component scores are shown in Figure 3.4 Siebenhaar also noted that avapritinib is the first treatment that can lead to improvement and even resolution of skin lesions in patients with ISM, which can have a big impact on patients’ QoL. After 24 weeks of avapritinib treatment versus placebo, the surface area of skin lesions reduced by a mean of 37% versus 2% in the most affected skin region, and the colour of the skin lesions lightened in 86% versus 0%, respectively.40
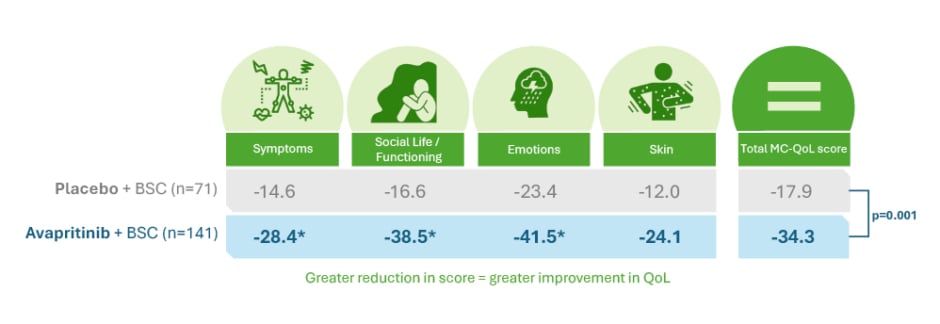
Figure 3: Percentage change in mean Mastocytosis Quality of Life questionnaire component score from baseline to Week 24.4,37
*p≤0.05
BSC: best supportive care; MC-QoL: Mastocytosis Quality of Life questionnaire; QoL: quality of life.
Siebenhaar highlighted the favourable safety profile for avapritinib at 25 mg once daily after 24 weeks of treatment; oedemas (including oedema peripheral, face oedema, and periorbital oedema) were the most common adverse event (AE) in the avapritinib group (with an incidence ≥2 times that of placebo, not thought to be related to underlying ISM), occurring in 8.5%, 7.1%, and 6.4 %, respectively, of patients receiving avapritinib, and 4.2%, 1.4%, and 2.8%, respectively, of patients receiving placebo.4 The majority of AEs were of Grade 1–2 (70% versus 72%, for avapritinib versus placebo, respectively), and the frequency of serious AEs was lower in the avapritinib than the placebo group (5% versus 11%, respectively).4 AEs resulting in treatment discontinuation occurred in just 2% of patients treated with avapritinib and 1% of patients receiving placebo.5 Importantly, no new safety concerns were observed during the longer-term, open-label evaluation (median follow-up of 18 months), with the most common treatment-related AEs consistent with those already reported.41
Precision Medicines in Clinical Development*
*Note that these medicines are not approved for ISM in the European Union (EU).
Bezuclastinib
Bezuclastinib is an investigational, selective KIT D816V TKI currently being assessed in the Summit clinical study (NCT05186753).42 Summit is a randomised, double-blind, placebo-controlled trial in adult patients with non-advanced SM (including ISM and smoldering SM) and inadequate symptom control despite BSC.5 In Part 1b of the study, patients were randomly assigned to 100 mg or 150 mg once daily of bezuclastinib plus BSC (each n=11), or to placebo plus BSC (n=12).43
Over the initial 12 weeks of treatment (data cutoff, 18 December 2023), bezuclastinib 100 mg was associated with significant symptom improvement, with a 41% mean improvement in MAS from baseline to Week 12, compared with a 21% mean improvement for placebo. Half (50%) of patients treated with 100 mg bezuclastinib achieved ≥50% improvement in MAS compared with no placebo patients.43 In addition, patients reported a significant improvement in QoL with MC-QoL total score with bezuclastinib 100 mg versus placebo (-24.9 versus -12.4, p=0.046).43
Bezuclastinib also elicited reductions across markers of mast cell burden.43 Of those patients with baseline tryptase ≥20 ng/mL, nearly all patients treated with bezuclastinib achieved <20 ng/mL (100% on 100 mg, 89% on 150 mg, and 0% on placebo). Among patients with detectable KIT D816V at baseline, ≥50% reduction in KIT D816V VAF was achieved by 100% of patients on 100 mg bezuclastinib, 89% on 150 mg bezuclastinib, and none on placebo). Among patients with evaluable BM, ≥50% reduction in BM mast cells was achieved by 86% of patients on 100 mg bezuclastinib, 78% on 150 mg bezuclastinib, and 40% on placebo.
The early data, after 12 weeks of treatment, show an encouraging safety and tolerability profile for bezuclastinib at 100 mg, with the majority of treatment-related AEs being low grade, no serious AEs and no dose reductions due to AEs.
Elenestinib
Another investigational TKI that selectively targets KIT D816V, elenestinib, is being assessed in the HARBOR clinical study (NCT04910685).44 HARBOR is a randomised, double-blind, placebo-controlled trial in adult patients with ISM and inadequate symptom control despite BSC.6,44 In Part 1 of the study, patients with a moderate-to-severe symptom score (based on ISM-SAF TSS) were randomly assigned 3:1 to 25 mg, 50 mg, or 100 mg once daily of elenestinib plus BSC (n=10, n=10, and n=9, respectively), or to placebo plus BSC (n=10).6
Over the first 12 weeks of treatment, clinically meaningful symptom improvement was observed for all dose cohorts; mean reduction from baseline in ISM-SAF TSS was 22.2% for placebo, 28.5% for elenestinib 25 mg, 31.8% for elenestinib 50 mg, and 33.6% for elenestinib 100 mg.6,45
Biomarkers of mast-cell burden also improved in a dose-dependent manner. Patients receiving elenestinib at 25 mg, 50 mg, and 100 mg versus placebo showed a mean percent changes from baseline for serum tryptase of 15.4%, -50.9%, and -68.4% versus +3.3%, for KIT D816V VAF of -37.5%, -70.4%, and -77.0% versus -2.5%, and for BM mast cells of -22.6%, -28.1%, and -57.9% versus -20.0%.6,45
After a median duration of treatment of 22 weeks, elenestinib was generally well tolerated at all dose levels. There were no treatment-related serious AEs, or AEs that led to drug discontinuation.45
CONCLUSION
Overall, the emerging precision medicine approaches targeting the primary driver of ISM, KIT D816V mutation, demonstrated encouraging efficacy and favourable safety profiles in patients with ISM, with improvements in symptom severity, mast-cell burden and QoL.





