Meeting Summary
This symposium took place during the 2018 meeting of the European Academy of Allergy and Clinical Immunology (EAACI). Focussing on the fundamental issues of suboptimal management of patients with cow’s milk protein allergy (CMPA), the speakers discussed key themes for optimising management. Prof Madrazo-de la Garza evaluated the challenges of diagnosis and management of CMPA in infants. Nonspecific symptoms, indicative of other conditions, mean that CPMA is often misdiagnosed as lactose intolerance, a rare condition in infants. Increased awareness of CMPA symptoms and a clear distinction from lactose intolerance may facilitate earlier, accurate diagnosis and implementation of appropriate dietary interventions. Dr Nutten followed by exploring variability in the composition of commercialised extensively hydrolysed formulas (eHF) intended for the management of CMPA and the associated potential clinical impact. Large variations in peptide profiles and residual allergenicity reflect a lack of definition for eHF composition. Although the clinical trials required to confirm the efficacy of eHF by demonstrating tolerance in >90% of infants with CMPA are performed, composition analyses for characterisation, quality control, and reproducibility are crucial for ensuring safe and suitable products throughout the product lifecycle. Prof O’Mahony concluded the meeting by focussing on the importance of the gut microbiome in food allergy. The establishment of a stable gut microbial community closely tracks host growth and immune development. Delayed or altered establishment leads to microbiome immaturity, which has been associated with an increased risk of food allergies. Nutritional strategies, such as the use of eHF containing lactose, to support microbiome development complement existing CMPA treatment.
Knowledge Gaps in Diagnosing and Managing Cow’s Milk Protein Allergy
Professor José Armando Madrozo-de la Garza
CMPA is the most common food allergy in infants, yet diagnosis is challenging due to nonspecific symptoms. Limited recognition by healthcare professionals worldwide leads to misdiagnosis and unnecessary nutritional interventions. Accurate dietary advice is important for the effective management of CMPA and the optimal development of affected patients.1
Self-overestimation of food allergies is common. Worldwide, 28% of people self-identified with an allergy; however, by skin prick test, only 8% had a confirmed food allergy and by double-blind placebo-controlled challenge test this reduced to only approximately 3%.2,3 The EuroPrevall birth cohort study followed 12,049 newborn babies from nine European countries over a 5-year period. The results showed variation in the proportions of self-reported food allergy, from 5–8% in Spain to 30% in Germany.4
Despite global variation in diet, the three most common food allergens worldwide are cow’s milk, egg, and seafood.5 Among adults, the most common adverse reaction to food is lactose intolerance. Approximately half to two-thirds of the world’s adults have primary lactose intolerance, but it is very rare in children aged <5 years.6 Secondary lactose intolerance can be seen in infants, although still rare, and is usually temporary and caused by transient lactase deficiency as a result of small bowel injury.7 Low-grade lactose malabsorption is a natural physiological phenomenon in breastfed and formula-fed newborns and young infants, enabling the production of softer and more acidic stools, which promotes gut microbiome development and increases production of short chain fatty acids.6
Lactose intolerance is due to an enzymatic deficiency, whereas CMPA is regulated by the immune system, either IgE-mediated or non-IgE-mediated.8 Symptoms common to both lactose intolerance and CMPA include diarrhoea, perianal rash, and inability to gain weight; however, CMPA is also characterised by vomiting, eczema, and occasionally rectal bleeding.6,7 A recent European survey uncovered major deficits in the management of CMPA, including limited knowledge of appropriate diagnostic tests, use of elimination diets, and optimal selection of speciality formula for management of non-breastfed infants.9
A comprehensive survey has recently been conducted to identify misconceptions about CMPA and lactose intolerance among healthcare practitioners across various international clinical settings.10 The study aimed to understand the clinical practice of primary healthcare providers treating non-breastfed patients with CMPA or lactose intolerance. Access to local resources and tools for the diagnosis and treatment of CMPA and lactose intolerance were evaluated and knowledge gaps identified.
The survey was conducted in a number of countries worldwide between January and November 2017.10 Participants were asked 12 multiple-choice questions on CMPA and lactose intolerance, questions relating to two clinical case scenarios, and 10 questions on educational needs. Over 50% of the 1,663 respondents had >10 years’ clinical practice experience. Most were general practitioners or general paediatricians working in tertiary care or private practice. Over 60% of the respondents felt confident in distinguishing CMPA from lactose intolerance; however, misconceptions were identified, including overestimation of the prevalence of lactose intolerance among infants. Although 59% of respondents identified eHF as the first-line treatment for managing CMPA, there was uncertainty about the use of lactose-free or lactose-containing eHF, and only 23% correctly identified an amino acid-based formula as an appropriate treatment for cases with anaphylaxis.
There were differences in perceived characteristic symptoms of IgE-mediated CMPA, non IgE-mediated CMPA, and lactose intolerance (Figure 1).10 For IgE-mediated CMPA, the most common symptoms identified were atopic dermatitis, diarrhoea, hives, vomiting, and abdominal pain, although there were some differences in responses by country. For non-IgE-mediated CMPA, the most common symptoms were identified as diarrhoea, abdominal pain, poor weight gain, abdominal bloating, feeding difficulties, and vomiting. Most responses considered only symptoms related to gastrointestinal manifestations rather than wider immune responses, with only a small proportion of respondents considering atopic dermatitis or urticaria as potential symptoms. Although some differences were noted among the countries in identification of primary symptoms, diarrhoea was widely recognised as the leading or second-leading sign or symptom. For lactose intolerance, respondents in all countries identified diarrhoea as the main symptom. Symptoms associated with immune response, such as urticaria, atopic dermatitis, and anaphylaxis, were only mentioned by a small proportion of respondents.
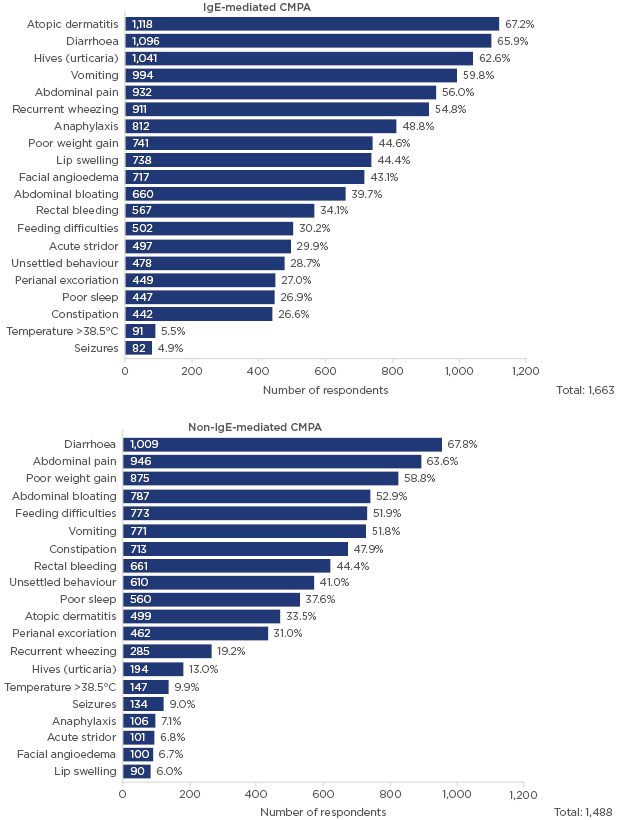
Figure 1: Which clinical signs or symptoms do you consider characteristic of IgE-mediated CMPA and non-IgE-mediated CMPA?
This survey was conducted in >40 countries with 1,663 respondents; multiple answers were permitted from each participant for this question.
CMPA: cow’s milk protein allergy.
Survey participants were also asked to review two clinical cases and suggest the appropriate formula for management. Clinical Case A was a full-term, vaginal-delivered male infant aged 4 months, exclusively breastfed to 2 months of age, presenting with persistent diarrhoea and eczema. Cow’s milk-based formula was introduced at 2 months, followed by development of mild-to-moderate eczema across his body and face, increased regurgitation, loose bowel movements (up to six times a day without visible blood), mild perianal excoriation, and weight loss (from the 25th to the 10th percentile). A total of 40% of survey participants selected initiation of eHF without lactose as the appropriate course of treatment, which was considered the correct choice, as secondary lactose intolerance was indicated by diarrhoea and perianal excoriation.
Clinical Case B was a full-term male infant delivered by caesarean section, aged 5 months and exclusively breastfed, without introduction of solid foods, presenting with generalised urticaria after drinking approximately 60 mL of cow’s milk-based formula. He also vomited once, became lethargic and floppy, and was taken to hospital for treatment and observation, where formula was avoided. He had moderate atopic eczema that started from 2 months of age. Most respondents selected an eHF for treatment in this case: 27% selected eHF without lactose and 27% selected eHF with lactose. Amino acid-based formula, the most appropriate option considering the anaphylactic reaction, was selected only by 24% of respondents but was the top choice for physicians in some countries.
The survey also contained questions about diagnostic procedures for identifying IgE-mediated and non-IgE-mediated CMPA, and lactose intolerance. Overall, identification of serum IgE specific to cow’s milk and skin prick testing were the most common tools used to diagnose IgE-mediated CMPA. For non-IgE-mediated CMPA, many participants recommended either no diagnostic test or a home challenge to cow’s milk.
When asked to consider awareness and education, >60% of respondents felt confident about their skills in diagnosing and managing CMPA, but 82% were interested in receiving further training. Almost half of respondents considered primary lactose intolerance to be common in infancy, despite it being rare in children <5 years old.
Overall, the survey results indicate that clinical recognition and management of CMPA versus lactose intolerance in infancy still poses clinical dilemmas. Significant educational gaps about the diagnosis and treatment of CMPA and lactose intolerance have been identified in several regions globally. There is much room for improvement and a need for targeted education and training to promote evidence-based clinical practice, change perceptions, and prompt physicians to suspect and test for CMPA in infants.
How to Define Extensively Hydrolysed Formula for the Management of Cow’s Milk Protein Allergy
Doctor Sophie Nutten
CMPA is the most common food allergy in infants, affecting 2–3% children worldwide.7 Dr Nutten reported that most children with CMPA have ≥2 symptoms: 50–70% have skin symptoms, 50–60% have gastrointestinal symptoms, and 20-30% have airway symptoms.11,12 Severe and life-threatening symptoms may occur in 10% of children.2 Management of these patients focusses on the avoidance of milk proteins and prompt recognition and treatment of allergic reactions resulting from accidental exposure.
Breast milk remains the gold standard for feeding infants with CMPA, with speciality formulas recommended when breastfeeding is not possible. CMPA management guidelines recommend the use of eHF as the first nutritional intervention unless there is an anaphylactic reaction to the original cow’s milk product, in cases of eosinophilic oesophagitis, or if the eHF is not tolerated.2,13-16 eHF contains cow’s milk peptides. The key properties of eHF are good safety and tolerability for most babies with CMPA and nutritional completeness to support growth and development. Despite these common goals, eHF have different compositions, with variation observed in carbohydrate sources (lactose versus no lactose), lipid profiles, protein sources, and also in the hydrolysis process. The hydrolysation process involves breaking down larger milk proteins to form small peptides, based on the rationale that peptide size is related to allergenicity. Theoretically, to bind with cell membrane-bound IgE, peptides should be approximately >1,500 Da in size (approximately 15 amino acids), and to crosslink IgE molecules and induce an immune response, they must be >3,000 Da in size (approximately 30 amino acids).17
CMPA management guidelines include definitions of eHF composition;2,13-16 however, there is a lack of consistency between guidelines when it comes to the definition of peptide size. There are some proposed specifications for peptide size, but thresholds are differently defined, with some guidelines stipulating a maximum size of 3,000 Da and others stipulating the largest proportion of peptides should be <1,000 Da.7,18 Permitted proportions of differently sized peptides (i.e., how much of the protein content must be below the threshold size) are also not consistently defined. This variation is reflected in the heterogeneity of composition of different eHF, which is thought to be the basis of observed tolerability differences.19
Providing information to physicians on the degree of hydrolysis and residual allergenicity in eHF would improve selection of optimal formulas. To contribute to this objective, Nestlé Health Science, Vevey, Switzerland, profiled samples of eHF to assess variations in eHF composition around the world (manuscript in preparation).20 Results presented during this symposium included samples available for sale collected from 10 countries (UK, the Netherlands, Sweden, Finland, Russia, China, Czech Republic, France, Spain, and Mexico), including a variety of manufacturers as well as different batches of the same formula. All formulas tested were eHF intended for the management of CMPA.
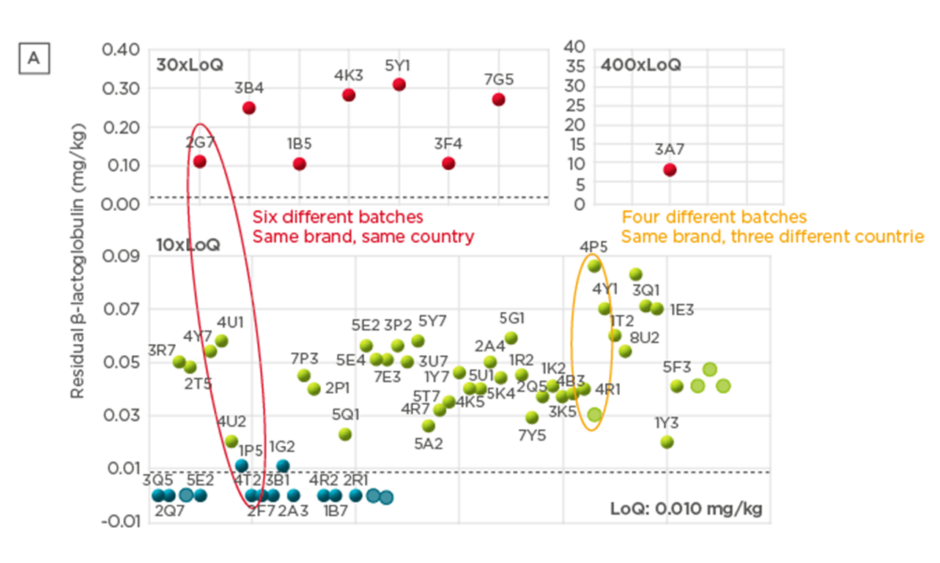
A battery of analytical tests were conducted to evaluate the composition of the different formulas. A high degree of variation was observed both in β-lactoglobulin content and in residual casein content (67 samples tested; Figure 2). Importantly, batch-to-batch variation was observed, both across and within countries. Significant variability in peptide molecular weight distribution was also observed (Figure 3), with the proportion of peptides >1,200 Da (critical size for IgE binding) ranging from 1.0–36.0% (66 samples).20 A large variation (<0.2–7.0%) was observed in the proportion of peptides >3,000 Da (critical size for IgE crosslinking, leading to symptoms). The European Commission Directive 2006/141/EC limited the content of immunoreactive proteins to <1% of total protein in hydrolysates.21 If we consider peptides >3,000 Da as immunoreactive, in this analysis, the proportion of peptides >3,000 Da was >1% in 3 of the 10 samples analysed. Finally, residual in vitro β-lactoglobulin allergenicity22,23 was found in 60% of the 10 eHF samples analysed, including 7 of the 8 whey-based eHF. Overall, the analyses showed wide variation in the composition of the peptide fraction of commercially available eHF, even between batches of the same brand, indicating that reproducibility is not well controlled during manufacturing.
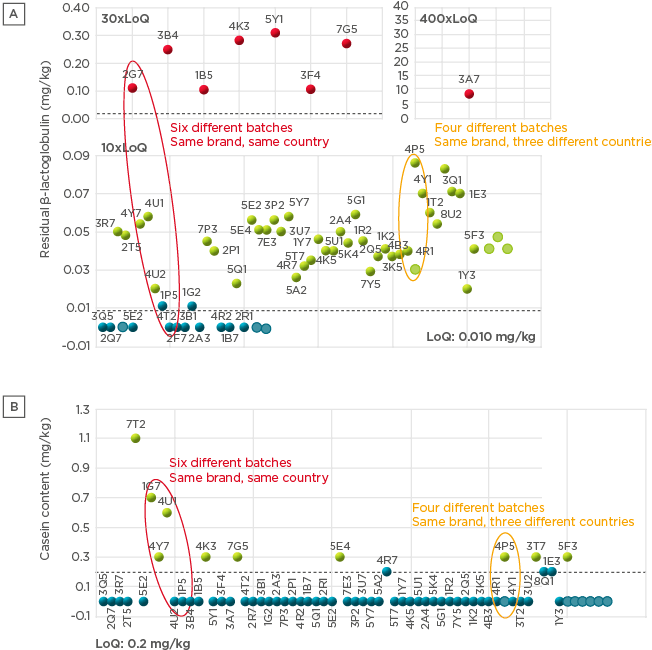
Figure 2: Residual β-lactoglobulin content (A) and residual casein content (B) in commercially available extensively hydrolysed formula samples.
LoQ: limit of quantitation.
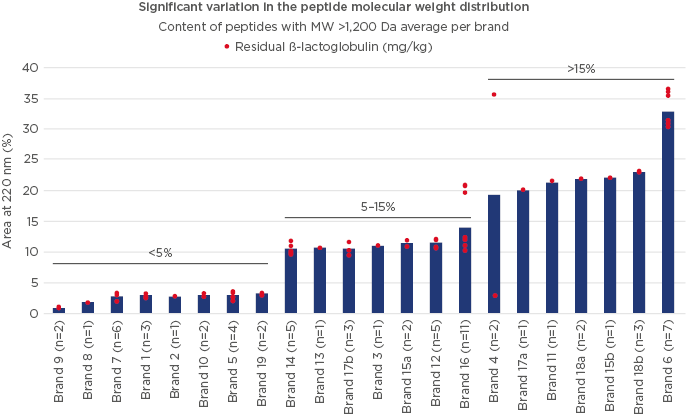
Figure 3: Variation in molecular weight distribution and residual β-lactoglobulin content in different samples of extensively hydrolysed formula.
MW: molecular weight.
All guidelines recommend that clinical trials should be conducted to determine suitability and safety of eHF, with a general rule that eHF must be tolerated by >90% of infants.2,13-16 However, clinical trials generally only provide a snapshot: a single test based on a single production batch. As wide variation has been demonstrated between different batches of the same formula, reproducibility in day-to-day manufacturing is key to ensure product safety and quality, supported by diligent processes.
Currently, no aligned and actionable definitions are in place to characterise and monitor the quality of eHF, which is reflected in the wide variation observed in the degree of hydrolysis of commercial eHF. More rigorous, globally applied standard definitions of eHF that can be universally applied by manufacturers are necessary. Analytical methods are available to characterise eHF, yet they are not standardised nor proven predictive of a potential allergic reaction. As shown in this study, there is good correlation between peptide molecular weight and allergenicity, which suggests that a high degree of hydrolysis may be preferable. Clinical trials remain important, as a successful, well-designed trial can establish the suitability of formula composition, but a strict quality control of the entire production process is crucial to guarantee consistent composition of the final product and eliminate contamination risks.
The Gut Microbiome and its Role in Early Immune Development and Allergies
Professor Liam O’Mahony
Recent investigations have explored the impact of the gut microbiome on the immune system, including its role in sensitisation to allergens and associated short and long-term effects.24 Every cell in the mucosal immune system communicates with those around it and responds to the presence of bacteria and secretion of bacterial metabolites.25 Induction of T regulatory cells by gut microbiota is important for oral tolerance to allergens, as well as wider general tolerance.26 The beneficial effects of gut microbiota are apparent from the earliest days of life but vary depending on the strain of bacteria. For example, certain Bifidobacteria can induce a T regulatory cell response to allergens in germ-free mice, reducing the IgE response to a later challenge by allergens.27 The severity of the allergic response is associated with the types of bacteria present in the gut, with some types associated with a heightened response to allergens.28
Children with CMPA have altered microbiome composition and metabolism compared with children who do not have CMPA.29 In addition, the gut microbiome in children whose CMPA spontaneously resolves is different compared with those in whom allergy persists.30 Surgery and exposure to antibiotics early in life increase the risk of developing a food allergy, but early administration of probiotics in these patients can reduce the risk of CMPA.31 Therefore, it may be possible to target probiotics to children at greater risk of gut dysbiosis, and further research in this area is warranted.
Humans are not born with a complete gut microbiome; rather, this is acquired gradually, with development of a mature microbiome requiring 2–3 years.32 Type of birth, diet, and antibiotic exposure affect microbiome development during this time.33-35 In particular, babies born vaginally have more complex and varied microbiomes than those delivered by caesarean section, with strain-matching showing direct acquisition of some bacterial strains from the mother.33 Gut dysbiosis due to type of birth can persist into childhood and may have longer-term consequences,33 and Prof O’Mahony suggested that inherited strains of gut bacteria may be progressively lost in multiple generations of babies born by caesarean section.
Microbiome development is also heavily influenced by nutrition, including the timing of introduction of yoghurt, fruit, and vegetables into the diet. Balanced nutrition in early life can reduce the risks of developing a food allergy.36 Short chain fatty acids are immunoregulatory and bind to G-protein-coupled receptors, promoting T regulatory cell and T helper cell responses. These molecules are microbial metabolites from dietary fibre, which is fermented by the gut microbiota, and are also present in some foods such as butter and yoghurt, which contain the short chain fatty acid butyrate. (Roduit and Frei et al. Manuscript submitted.) Children with higher butyrate levels at 1 year of age have fewer allergies by the age of 6 years than those with lower levels. Lactose, which is present in breast milk, is also an important early life prebiotic. It has been shown that children with CMPA consuming an eHF with lactose have significantly higher counts of Bifidobacteria and lactic acid bacteria as well as significantly higher levels of short chain fatty acids than children consuming an eHF not containing lactose.37 A lack of lactose in specialised formula for children with CMPA could have a detrimental effect on microbiome development and associated response to food allergens.37 Obesity also affects the microbiome and a higher BMI is associated with greater sensitisation to IgE and food allergies, although the effects are not thought to be direct.38
Exposure to antibiotics can significantly delay maturation of the gut microbiome.33,34 The type of antibiotic, length of exposure, and administration route all influence microbiome development. Although there are conflicting data about the use of antibiotics and the development of food allergies, it is feasible to suggest that antibiotics may modify the microbiome, leading to increased response to, for example, cow’s milk protein.39
Several opportunities are available for clinical practice interventions to improve food tolerance and reduce allergies via actions on the gut microbiome.40 Reducing the number of elective caesarean sections would mitigate microbial dysbiosis and potentially reduce the risk of allergic immune responses and inflammation. Reduction in indiscriminate use of antibiotics during infancy and the perinatal period may prevent delayed maturation of the gut microbiome. Minimising inappropriate use of lactose-free formula for infants could have beneficial effects on the microbiome, possibly improving tolerance to food allergens. Finally, an increase in dietary intake of fermentable fibre to increase short chain fatty acids produced by microbial fermentation might reduce allergic responses.
Conclusion
To conclude, the management of patients with CMPA is a challenge but can be optimised. Three fundamental themes explored during this symposium could be instrumental in improving the management of infants with CMPA. First, targeted education and training is essential to improve early recognition of symptoms and to minimise misdiagnosis, such as lactose intolerance. Second, manufacturers of infant formula should consider focussing on quality control and reproducibility of formula production processes to guarantee consistent composition. Heterogeneity of available eHF is a challenge and the development of standardised global guidelines and definitions for eHF composition is necessary to ensure that physicians can select appropriate formulas for their patients. Finally, the role of the gut microbiome in sensitivity and tolerance to food allergens and the prebiotic role of lactose should be considered. Inappropriate use of lactose-free eHF for infants who are not lactose intolerant could have a detrimental effect on microbiome establishment, particularly in children already at risk of delayed microbiome maturation.