Meeting Summary
Asthma is a heterogeneous disease with multiple phenotypes, caused by a complex interplay of inflammatory pathways. Up to 70% of patients with asthma have Type 2 inflammation, characterised by the presence of interleukin (IL)-4, IL-5, and IL-13. Uncontrolled persistent asthma represents a considerable disease burden associated with a higher rate of exacerbations, more frequent hospitalisations, greater oral corticosteroid (OCS) use, more impaired lung function, reduced health-related quality of life (QoL), and Type 2 inflammatory comorbidities versus controlled asthma. There remains an unmet need for new therapies for patients with uncontrolled persistent asthma. Several agents targeting mediators of Type 2 inflammation are in clinical development for severe asthma, including prostaglandin D2 receptor 2 (DP2)/chemoattractant receptor-homologous molecule expressed on Th2 (CRTh2) antagonists and monoclonal antibodies (mAb) that specifically bind IL-33, IL-25, thymic stromal lymphopoietin (TSLP), and IL-4 receptor (IL-4Rα). Dupilumab blocks signalling of IL-4 and IL-13 and is under investigation in various diseases driven by Type 2 inflammation. In Phase III clinical trials in patients with uncontrolled, persistent asthma, dupilumab was well tolerated and demonstrated significant efficacy versus placebo in reducing the rate of asthma exacerbations, and improving lung function, asthma symptoms, and QoL. This article summarises the proceedings of a symposium held at the European Academy of Allergy and Clinical Immunology (EAACI) 2018 Congress, which brought together an international faculty of experts to explore current understandings of asthma pathophysiology, with particular focus on Type 2 inflammatory pathways, and to provide an overview of current therapies, unmet medical needs, and the potential role of emerging biologics in the treatment of uncontrolled persistent asthma.
Introduction
Asthma is defined by the Global Initiative for Asthma as a history of respiratory symptoms such as wheeze, dyspnoea, chest tightness, and cough that vary over time and intensity, together with variable expiratory airflow limitation.1 It is a common chronic respiratory disorder that affects an estimated 358 million people worldwide,2 and is associated with a substantial socioeconomic burden. It is now acknowledged to be a heterogeneous disease with multiple phenotypes, based on distinct pathophysiological mechanisms.1 Comorbidities are common, which can have a significant effect on patients’ exacerbations, symptom control, and QoL.3 Importantly, up to 56% of patients continue to have uncontrolled persistent asthma, despite the availability of effective asthma treatments and evidence-based management guidelines.4-6
Asthma Heterogeneity is Caused by a Complex Interplay of Inflammatory Pathways
Chronic airway inflammation is the main pathophysiological feature of asthma, together with goblet cell hyperplasia, enhanced mucus production, and smooth muscle contractility abnormalities, as well as airway remodelling. The clinical manifestations of these processes result in airway hyper-responsiveness, airflow obstruction, decreased lung function, and exacerbations.7 The heterogeneity of asthma is likely the result of a complex interplay of inflammatory pathways that involve multiple cytokines and inflammatory cells.7 Type 2 inflammation in the airway is characterised by the presence of IL-4, 5, and 13, which are produced by Type 2 helper T (Th2) cells and innate lymphoid cells in response to allergens, infectious agents, irritants, and pollutants.7
The interplay between innate and adaptive cells and mediators in Type 2 inflammation underpins asthma pathophysiology,7 with Type 2 cytokines playing unique but overlapping roles. IL-13 contributes to goblet cell hyperplasia, increases production of MUC5AC (associated with a more adherent type of mucus), induces production of the eosinophil chemoattractant eotaxin, and induces airway smooth muscle contractility and proliferation.8,9 IL-4 and IL-13 contribute to epithelial barrier disruption by increasing epithelial permeability, and are central to airway remodelling.10-12
Type 2 Inflammation is Associated with Multiple Asthma Phenotypes
Asthma driven by Type 2 inflammation (Type-2 high) encompasses allergic (childhood onset) and eosinophilic (adult-onset) phenotypes.13 These are associated with well-recognised biomarkers such as the presence of blood and sputum eosinophils, elevated fractional exhaled nitric oxide (FeNO; an indirect measure of pulmonary inflammation), serum immunoglobulin (Ig)E, and serum periostin.14 High levels of sputum and blood eosinophils, and FeNO, are associated with uncontrolled and/or more severe asthma and the risk of exacerbations.15,16 Approximately 50–70% of patients with asthma have Type 2 inflammation.17,18 Both IL-4 and IL-13 are key players in Type 2-mediated inflammation in asthma, and have central roles in IgE synthesis, eosinophil recruitment, mucus secretion and airway inflammation, hyper-reactivity, and remodelling.19 Non-Type 2 (Type-2 low) inflammatory pathways are not well understood, but may include those associated with smooth muscle-mediated factors, obesity, infection, or neutrophilia.20
Burden of Uncontrolled Persistent Asthma
Patients with severe, persistent asthma comprise around 5–10% of the total asthma population, but account for >80% of the total direct healthcare costs of asthma.21,22 Uncontrolled persistent asthma is a complex disease state associated with a high rate of exacerbations, frequent hospitalisations, high oral OCS use, impaired lung function, reduced health-related QoL, and Type 2 inflammatory comorbidities.1,15,23,24 Exacerbations can be potentially life-threatening, requiring medical intervention in the form of an emergency department visit or admission to hospital,25 and are thus linked to higher morbidity, greater risk of mortality, and higher treatment costs.26 Of note, it has been reported that the total cost of managing an exacerbation increases with disease severity and is particularly driven by prior exacerbations.27 Additionally, there are concerns regarding long-term, regular OCS use, because of potential systemic adverse effects on growth, adrenal function, and bone mass and associations with conditions such as hypertension, Type 2 diabetes mellitus, and gastrointestinal bleeding.28-30
The burden of uncontrolled persistent asthma driven by Type 2 inflammation is particularly high. Simultaneous increases in FeNO and blood eosinophil biomarkers are associated with more frequent exacerbations.15 Other Type 2 inflammatory diseases, notably allergic rhinitis and chronic rhinosinusitis with nasal polyps, are often comorbid with uncontrolled persistent asthma.31,32 An analysis of data from the UK Optimum Patient Care Research Database showed that both conditions are independent predictors of asthma exacerbations,33 while data from the Severe Asthma Research Program (SARP) demonstrated that sinusitis is associated with an increased exacerbation rate in patients with severe asthma.34
Challenges in the Management of Uncontrolled Persistent Asthma
The Global Initiative for Asthma 2018 guidelines advise a stepwise approach to the use of treatments to achieve asthma control.1 In patients in whom asthma is severely uncontrolled or with acute exacerbations, a short course of OCS is recommended as an add-on to regular treatment. However, OCS are associated with a variety of significant long-term side effects in patients with severe asthma, which include osteoporosis, hypertension, obesity, Type 2 diabetes, gastrointestinal ulcers/bleeds, fractures, cataracts, muscle weakness, back pain, and bruising.29,30 Unsurprisingly, long-term OCS use remains a concern and, in a recent large survey in patients with asthma (N=2,003), approximately one-third and one-half of respondents from Europe and Canada, respectively, cited it as a worry.35 Therefore, the use of steroid-sparing treatments is important, where possible, to minimise side effects and improve patient outcomes.
There is a lack of evidence to support an inhaled corticosteroids dose increase and other non-biologic controller therapy add-ons (treatment escalation strategies) in patients with uncontrolled asthma. In a USA real-world healthcare claims database study of patients who initiated a treatment escalation strategy, uncontrolled asthma was experienced by 41.5% and 41.0% before and after treatment escalation, respectively.36 Outcomes before the implementation of an escalation strategy were similar 1 year later. To address the considerable and remaining unmet need for new treatment approaches beyond escalation, a number of biologic therapies targeting the Type 2 inflammatory pathway are approved or in development.
Overview of Current Evidence on Approved and Emerging Biologic Therapies Targeting Type 2 Inflammation
Four biologic therapies are currently licensed in the European Union (EU) and the USA for the treatment of asthma: omalizumab (approved for moderate-to-severe allergic asthma in patients aged ≥6 years), benralizumab and mepolizumab (approved for severe asthma in patients aged ≥12 years with an eosinophilic phenotype), and reslizumab (approved for severe eosinophilic asthma in patients aged ≥18 years).
Omalizumab is a humanised anti-IgE mAb that specifically binds free IgE in serum and can interrupt the allergic cascade by preventing the binding of IgE with its high-affinity FcεRI receptors on mast cells, antigen-presenting cells, basophils, and other inflammatory cells.37 In clinical and real-world studies involving children, adolescents, and adults, all with moderate-to-severe asthma, omalizumab treatment was well tolerated and was shown to significantly reduce asthma exacerbations, symptoms, and the need for inhaled corticosteroid and rescue medication use. QoL was also improved versus placebo or standard of care.38-41
The other three licensed biologics target IL-5 signalling, binding either IL-5 directly (humanised mAb: mepolizumab and reslizumab) or its receptor IL-5R (fully human mAb benralizumab), resulting in a depletion of blood and airway eosinophils and basophils.42 In children and adults with severe eosinophilic asthma, all three treatments significantly reduced rates of clinically significant asthma exacerbations by ~50% and significantly improved lung function, as shown by increases in mean forced expiratory volume in 1 second (FEV1) of 0.08–0.11 L versus placebo.43-49 Patients receiving mepolizumab or benralizumab also significantly reduced their OCS intake (by 50% and 75%, respectively),43,50 and improvements in asthma control and QoL were seen with mepolizumab or reslizumab treatment versus placebo.43,44,49 The safety profiles of all three therapies were comparable to that of placebo.43,46,47,49,50
Despite demonstrable efficacy, safety, and tolerability in clinical and real-world studies of patients with severe allergic or eosinophilic asthma, currently available biologic therapies have several limitations and are not suitable for, or effective in, many patients with asthma. Their use is often restricted to specific populations, leaving many patients ineligible for treatment. Evidence suggests that between 65% and 76% of patients with severe asthma may be ineligible for any approved biologic therapy, based on the eligibility criteria used.51 They do not show consistent activity across patients with a broad range of Type 2 biomarkers.52 A substantial proportion of patients remain suboptimally controlled, and there may be subphenotypes of Type 2-high asthma that do not respond to treatment.52,53 Current biologic therapies only partially inhibit Type 2 inflammation, and may therefore be less effective than biologics with a broader effect, and no available single biologic therapy can treat the full spectrum of Type 2 comorbid, inflammatory diseases (Table 1).
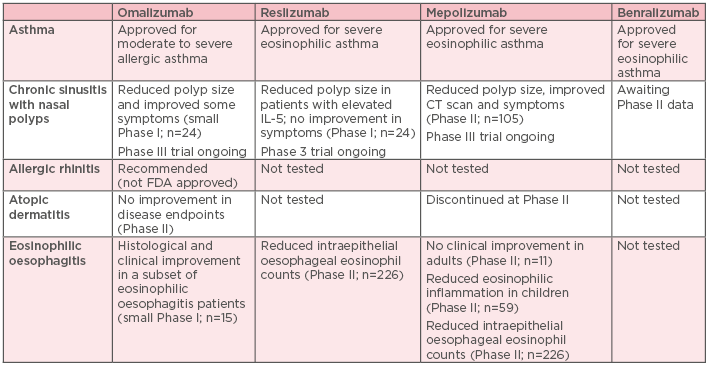
Table 1: Status of currently approved biologics for the treatment of common Type 2 comorbidities associated with uncontrolled persistent asthma.46-52,54-57
CT: computed tomography; FDA: U.S Food and Drug Administration; IL: interleukin.
To address these limitations, a variety of mAb and small molecules that target different mediators of, and pathways involved in, Type 2 inflammation other than IgE and IL-5/IL-5R are under investigation. Agents in Phase II clinical development include the anti-IL-33 mAb, AMG-282/RG6149 (for the treatment of mild atopic asthma), GSK3772847 and SAR44040/REGN3500 (for moderate-to-severe asthma), and ANB020 (for severe eosinophilic asthma). In addition, orally administered ADC3680/ADC3608B is a potent and selective antagonist of the DP2/CRTh2 for inadequately controlled asthma. Agents undergoing Phase III clinical testing for poorly controlled or uncontrolled, persistent or severe asthma include ABM125 (an anti-IL-25 mAb), fevipiprant (an orally administered competitive and reversible antagonist of DP2/CRTh2), tezepelumab (a human mAb that specifically targets the epithelial cell-derived cytokine, TSLP), and dupilumab (a fully human mAb that specifically binds to the alpha subunit of IL-4Rα).
Dupilumab will be the focus of the rest of this review, because it is being investigated in a broad range of clinical development programmes for diseases driven by Type 2 inflammation. It is already licensed for moderate-to-severe atopic dermatitis in adults, and, in addition to uncontrolled, persistent asthma, clinical studies are being conducted in paediatric atopic dermatitis (Phase III), chronic rhinosinusitis with nasal polyps (Phase III), and eosinophilic oesophagitis (Phase II). Dupilumab blocks signalling of IL-4 and IL-13, both of which bind to IL-4Rα and are key drivers of Type 2 inflammation.31
A 24-week, randomised, double-blind, placebo-controlled, parallel-group, pivotal, Phase IIb dose-ranging study58 evaluated subcutaneous dupilumab (200 mg or 300 mg every 2 [Q2W] or 4 [Q4W] weeks) as add-on therapy in patients aged ≥18 years with uncontrolled, persistent asthma on medium-to-high-dose inhaled corticosteroids plus a long-acting β2-agonist (N=769). The results showed that dupilumab significantly increased lung function (assessed by FEV1; p<0.01) and reduced severe exacerbations by ~60–81% versus placebo (p<0.05).58 Dupilumab treatment also significantly improved asthma control (least squares mean change in 5-item Asthma Control Questionnaire [ACQ-5] score from baseline to Week 24 of -1.49 and -1.45 for 200 and 300 mg dupilumab, respectively, versus -1.14 for placebo) and QoL (measured with the Asthma Quality of Life Questionnaire) and had a favourable safety profile.58 This study established the optimum dupilumab dosing regimen to be Q2W.
Phase III Clinical Studies of Dupilumab in Patients with Uncontrolled, Persistent Asthma: LIBERTY ASTHMA QUEST and VENTURE
The 52-week, randomised, double-blind, placebo-controlled, parallel-group, Phase III LIBERTY ASTHMA QUEST study59 randomised patients aged ≥12 years with uncontrolled, persistent, moderate-to-severe asthma (N=1,902) to subcutaneous dupilumab as add-on therapy (200 mg or 300 mg Q2W) or placebo. Primary endpoints included annualised severe asthma exacerbation rate and absolute change from baseline to Week 12 in FEV1 before bronchodilator use. Secondary endpoints included exacerbation rate and FEV1 in patients with a blood eosinophil count of ≥300 cells/mm3 and stratified by baseline FeNO, a measure of pulmonary inflammation.59
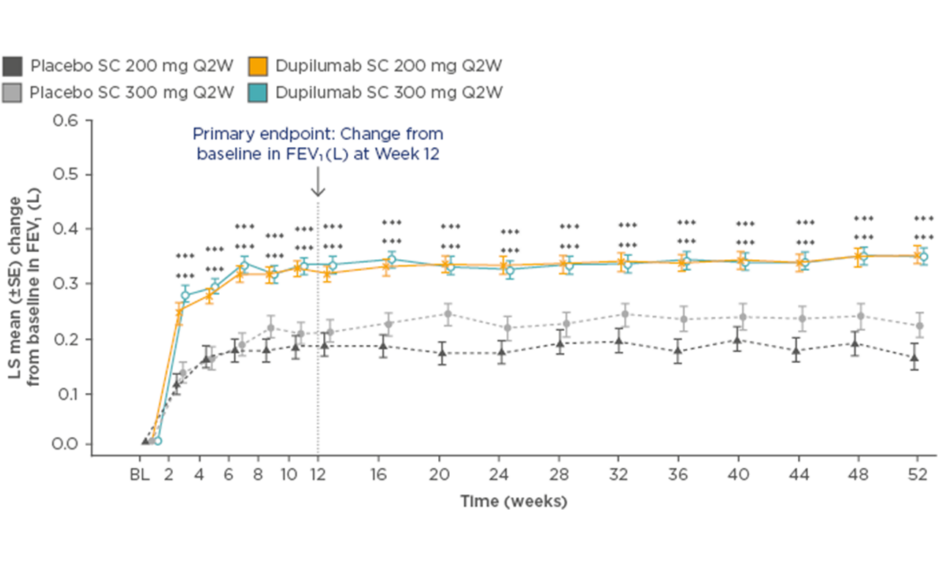
In the dupilumab 200 mg Q2W group, the annualised rate of severe asthma exacerbations was 0.46 (95% confidence interval [CI]: 0.39–0.53) versus 0.87 (95% CI: 0.72–1.05) for placebo, equating to a 48% reduction with dupilumab (p<0.001). FEV1 increased by 0.32 L at 12 weeks (difference versus matched placebo: 0.14 L; p<0.001), and this significant and rapid improvement in lung function was sustained for the remainder of the study (Figure 1). For both primary endpoints, similar results were seen with the 300 mg dose.59
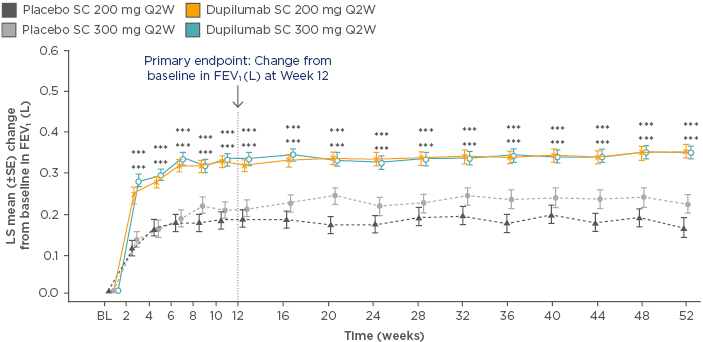
Figure 1: Least squares mean change from baseline in forced expiratory volume in 1 second over 52 weeks of treatment with dupilumab versus placebo.
***p<0.001 versus placebo
BL: baseline; FEV1: forced expiratory volume in 1 second; LS: least squares; Q2W: every 2 weeks; SC: subcutaneous; SE: standard error.
Adapted from Castro et al.59
Among patients with eosinophilic asthma, the annualised rates of severe asthma exacerbations were 0.37 (95% CI: 0.29–0.48) in the dupilumab 200 mg group versus 1.08 (95% CI: 0.85–1.38) for matched placebo and 0.40 (95% CI: 0.32–0.51) in the dupilumab 300 mg group versus 1.24 (95% CI: 0.97–1.57) for matched placebo. This equated to a reduction in severe asthma exacerbations of 66% and 67% with dupilumab 200 mg and 300 mg, respectively (p<0.001). In patients stratified by baseline FeNO, a greater benefit in exacerbation rate with dupilumab was observed in patients with higher FeNO: ≥50 ppb and ≥25 ppb FeNO levels were associated with significant reductions of 69–70% and 61–65% versus placebo for the 200 mg and 300 mg dupilumab doses (p<0.001), but no significant between-group differences were seen in patients with FeNO <25 ppb (Figure 2). Significantly greater improvements in lung function were also observed in patients with higher baseline eosinophil counts and FeNO levels. Hypereosinophilia was observed in some patients soon after starting treatment (4.1% [n=52] versus 0.6% [n=4] for dupilumab versus placebo, respectively).59
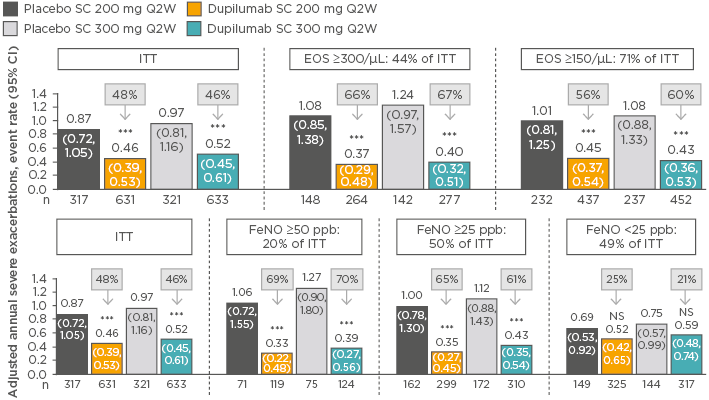
Figure 2: Rate of severe exacerbations in patients stratified by baseline blood eosinophil count or fractional exhaled nitric oxide level treated with dupilumab versus placebo (intent-to-treat population).59-61
***p<0.001 versus placebo.
CI: confidence interval; EOS: blood eosinophil count; FeNO: fractional exhaled nitric oxide; ITT: intent-to-treat; NS: nonsignificant; Q2Q: every 2 weeks; SC: subcutaneous.
In the 36-week, randomised, double-blind, placebo-controlled, Phase III LIBERTY ASTHMA VENTURE study,62 add-on therapy with dupilumab 300 mg Q2W significantly reduced the use of OCS, while simultaneously reducing severe exacerbations and improving lung function, in patients with corticosteroid-dependent severe asthma (N=210), irrespective of baseline blood eosinophil count. The primary endpoint of the study was percentage reduction in corticosteroid dose at Week 24; key secondary endpoints included proportions of patients at Week 24 with a reduction of ≤50% in corticosteroid dose, and with a reduction to a corticosteroid dose of <5 mg/day.62
At Week 24, the percentage change in corticosteroid dose was -70% versus -42% for the dupilumab and placebo groups, respectively (p<0.001), with 80% versus 50% of patients in these respective groups reducing their corticosteroid doses by ≥50% (p<0.001). In total, 69% of patients in the dupilumab group versus 33% in the placebo group had corticosteroid dose reductions to <5 mg/day (p<0.001), with 48% and 25% in the dupilumab and placebo groups, respectively, no longer requiring OCS (p=0.002). Despite these reductions in corticosteroid dosing, the severe exacerbation rate was 59% lower in the dupilumab group (95% CI: 37–74%), resulting in an FEV1 that was 0.22 L (95% CI: 0.09–0.34) higher. Compared with placebo, just over twice as many patients on dupilumab experienced injection-site reactions (9% versus 4%), and transient blood eosinophilia was observed in 14% of patients (versus 1% of placebo- treated patients).62
Dupilumab Efficacy in Type 2 Inflammatory Diseases
Type 2 inflammation is an important process leading to airway changes in asthma, including airflow obstruction, susceptibility to exacerbations, and persistent airway changes from remodelling. Dupilumab targets IL-4/IL-13 pathway generated-type inflammation. The effects of IL-4/IL-13 on asthma are comprehensive, and consequently the effects of dupilumab in asthma may be more profound than those of current treatments.
Dupilumab has also demonstrated efficacy in patients with comorbid Type 2 inflammatory diseases. In a randomised, double-blind, placebo-controlled, parallel-group, Phase II study63 in adult patients with symptomatic chronic sinusitis and nasal polyposis refractory to intranasal corticosteroids (N=60; n=35 with comorbid asthma), the addition of subcutaneous dupilumab to mometasone furoate nasal spray reduced endoscopic nasal polyp burden, compared with placebo plus mometasone alone after 16 weeks of treatment (least squares mean change in nasal polyp score: placebo: -0.3 [95% CI: -1.0–0.4]; dupilumab: -1.9 [95% CI: -2.5–-1.2]; p<0.001). Improvements in exploratory endpoints including percent predicted FEV1 and ACQ-5 score were also seen.63 The target population of dupilumab therefore appears to be much broader than that of existing IgE/IL-5-targeting biologics.
Conclusions
There remains an unmet need for new therapies to treat patients with persistent, uncontrolled asthma. Currently approved biologics have several limitations, and many patients are ineligible for treatment. A variety of agents that target mediators of Type 2 inflammation are in clinical development for severe asthma, including DP2/CRTh2 antagonists and mAb that specifically bind IL-33, IL-25, TSLP, and IL-4Rα. In Phase III clinical trials, dupilumab, an inhibitor of IL-4 and IL-13, was well tolerated and has demonstrated significant efficacy versus placebo at reducing the rate of asthma exacerbations, and improving lung function, asthma symptoms, and QoL. Due to the complexity of Type 2 inflammation in uncontrolled persistent asthma, assessing a wide panel of biomarkers would help identify pathway involvement and guide the future development of therapeutic interventions.
Click here to view the full symposium.