Meeting Summary
Cow’s milk allergy (CMA) currently ranks as one of the most common infant food allergies and requires timely diagnosis and appropriate management to mitigate the impact on growth and developmental outcomes and minimise patient/parent distress. During this symposium, chaired by Yvan Vandenplas, Emeritus at KidZ Health Castle, University Hospital Brussels, Belgium, leading experts in paediatric gastroenterology, allergy, and nutrition, discussed how best to navigate the CMA journey in clinical practice, from accurate differential diagnosis to nutritional optimisation. Annamaria Staiano, Professor of Paediatrics and Chief of the Department of Translational Medical Sciences at the University of Naples ‘Federico II’, Italy, discussed the complexities of differentiating disorders of gut–brain interaction (DGBI), previously known as functional gastrointestinal disorders, from CMA, and considered the potential role of the Cow’s Milk Related Symptom Score (CoMiSSTM) in ensuring infants follow the correct diagnostic pathway. Ralf Heine, Paediatric Gastroenterologist, Allergist, and Honorary Research Fellow at the Murdoch Children’s Research Institute in Melbourne, Australia, explored the immune-modulating effects of human milk oligosaccharides (HMO) and lactose on the gastrointestinal (GI) microbiota in infants, highlighting the importance of the early-life microbiome during the nutritional management of CMA. Rosan Meyer, Paediatric Dietitian and visiting Professor at both KU Leuven, Belgium, and the University of Winchester, UK, focused on the final phase of CMA management, the reintroduction of cow’s milk protein, and also examined new evidence to support the optimal timing and strategy for this key step in clinical practice.
DISTINGUISHING BETWEEN DISORDERS OF GUT BRAIN INTERACTION AND COW’S MILK ALLERGY IN CLINICAL PRACTICE
Annamaria Staiano
CMA is among the most frequently occurring food allergies in infancy, but pinpointing its precise prevalence in different age groups is difficult. According to British Society for Allergy and Clinical Immunology (BSACI) guidelines, CMA prevalence ranges from 1.8–7.5%, while data from the EuroPrevall project, a birth cohort study of 12,000 children, puts the overall figure at 0.54%, ranging between 0.3–1.0% across different countries.1,2
CMA is challenging to diagnose because it presents with a variety of clinical symptoms, most of which are non-specific and can be caused by a spectrum of different diseases and allergies.3 Dermatological, gastrointestinal, and respiratory symptoms are the most common and often occur in combination. Patients may also present with mixed IgE and non-IgE CMA symptoms. However, no single test or combination of tests is definitively diagnostic for CMA.
The complexities of diagnosing CMA are compounded by its similarity to DGBIs and the significant overlap of clinical symptoms. According to the Rome IV criteria, DGBIs in infants can be classified as: infant regurgitation, infant rumination syndrome, cyclic vomiting syndrome, infant colic, functional diarrhoea, infant dyschezia, and functional constipation.4 In a cross-sectional, multicentre, European study of nearly 1,700 infants aged 0–48 months, the prevalence of any DGBI (based on Rome IV criteria) in those aged 0–12 months was 24.7%, with 4% of subjects found to have multiple DGBIs at the same time.5 DGBIs therefore occur very frequently in infants and can coexist with other conditions that cause GI symptoms such as gastro-oesophageal reflux disease (GERD), eosinophilic oesophagitis, and other different conditions.6
Staiano went on to introduce CoMiSSTM, which is a well-established clinical awareness tool to support the diagnosis of CMA. The recent ESPGHAN position paper on diagnosis, management and prevention of CMA confirmed the role of CoMiSSTM as an awareness tool to alert healthcare professionals to the possibility of CMA in infants presenting with multiple symptoms, such as excessive crying, regurgitation, stool pattern changes, and skin and respiratory symptoms, especially if present in combination.7 Currently, the gold standard for confirming the diagnosis of both IgE- and non-IgE-mediated CMA is a double-blind, placebo-controlled food challenge (DBPCFC).7 However, as DBPCFCs are time-consuming and difficult to perform in daily practice, the open oral food challenge (OFC) is considered an acceptable alternative.7
Unfortunately, OFCs are often refused by parents, probably due to the fear of symptom relapse/recurrence after reintroduction of cow’s milk protein.7
In the clinical setting, distinguishing between CMA and DGBIs can prove particularly challenging. Staiano reiterated that the majority of infants with CMA and/or DGBI will present with a combination of symptoms. Given the high prevalence of infantile colic, regurgitation, and constipation, this combination can be causal or by coincidence, and the presence of one condition does not exclude the other. Clinical practice guidelines for the management of gastro-oesophageal reflux and GERD from birth to 1 year of age also indicate that CMA should always be considered in the differential diagnosis for infantile GERD, and a trial removal of cow’s milk protein is recommended.8 One study identified potential CMA in one-third of paediatric GERD cases, suggesting that CMA can mimic or aggravate the signs and symptoms of severe GERD during infancy.9 Staiano went on to consider the potential role of the CoMiSSTM awareness tool in helping to distinguish between DGBIs and CMA. CoMiSSTM recently celebrated the 10th anniversary of its publication and is now supported by a robust evidence base of 25 original studies including over 3,000 patients.10 When interpreting the CoMiSSTM score, a total score ≥10 is highly suggestive of CMA, while a score <6 indicates that symptoms are not likely to be CMA-related, and clinicians should therefore be encouraged to look for alternative causes. “But what about scores between 6–9?” Staiano asked. In a recent observational, real-world study of formula-fed infants aged 0–4 months presenting with at least two DGBIs (out of regurgitation, constipation, and crying), the mean CoMiSSTM score at baseline in this group of patients was found to be 6.46.11 Staiano, therefore, proposed a simple chart for distinguishing between DGBIs and CMA in clinical practice (Figure 1), in which a CoMiSSTM score <6 should be considered as normal, a score ≥10 is highly suggestive for CMA, and a score between 6–9 could indicate the presence of a DGBI.
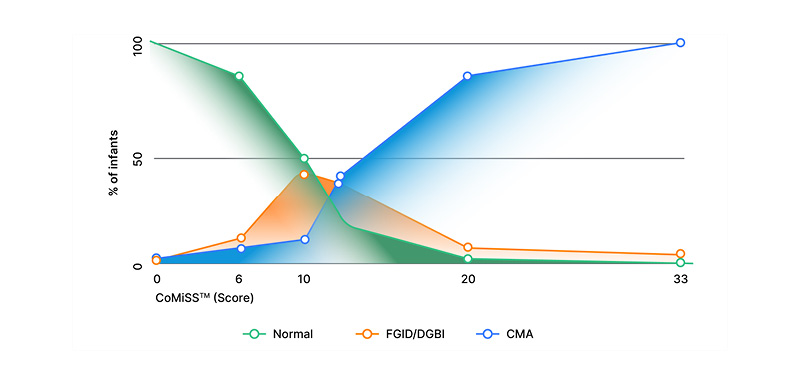
Figure 1: The potential role of CoMiSSTM in distinguishing between cow’s milk allergy and disorders of gut–brain interaction.
CMA: cow’s milk allergy; CoMiSSTM: cow’s milk-related symptom score; DGBI: disorder of gut–brain interaction;
FGID: functional gastrointestinal disorder.
In summary, the diagnosis of either CMA or DGBIs and the distinction between them is clinically challenging because of non-specific and overlapping symptoms. Both DGBIs and (non-IgE) CMA produce symptoms associated with the ingestion of cow’s milk protein, where elimination results in the disappearance of symptoms and reintroduction leads to relapse. In the diagnosis of CMA, there is already a large body of clinical evidence supporting the validity of CoMiSSTM as an awareness tool for healthcare professionals. Staiano, therefore, concluded that the CoMiSSTM tool may have an additional role in helping clinicians make the difficult differential diagnosis between CMA and DGBIs, thereby ensuring that all infants follow the correct diagnostic pathway. Across all countries, more education is also needed to ensure both the appropriate and timely diagnosis of CMA, and effective management of DGBIs if CMA is excluded.
UNLEASHING THE POWER OF THE MICROBIOME
Ralf G. Heine
The gut microbiome is an important modifier of disease risk from birth and throughout life. Heine explained that a healthy microbiome is enriched in bifidobacteria and healthy commensals, with a metabolome characterised by high levels of short-chain fatty acids (SCFA). This maintains mucosal barrier function effectively while reducing inflammation and modulating the activity of regulatory T cells. By contrast, dysbiosis is associated with a lack of bifidobacteria and overgrowth of proteobacteria, leading to a breakdown of barrier function that may increase permeability to bacterial and food antigens. Dysbiosis also creates a proinflammatory state characterised by a predominance of Th2 lymphocytes, which predisposes the patient to allergic sensitisation.12
The elements that modulate early gut microbiome development are multifaceted and include the mode of feeding, as well as other factors such as the environment, birth mode, and antibiotic use.13 In the large-scale birth cohort study, TEDDY, breast milk was identified as one of the most important determinants of early microbiome composition.14 As part of the nutritional management of CMA, it is therefore important to consider the infant microbiome in addition to other aspects of adequate nutritional intake, health, and development.
Heine described HMO, which consists of a complex mix of non-digestible carbohydrates, as the “single most important constituent” of breast milk with regard to supporting the infant gut microbiota and immune system development in early life. HMO, therefore, plays a pivotal role in infant nutrition and is also increasingly relevant to the management of CMA, as breast-milk identical HMO, notably 2’-fucosyllactose (2’-FL) and lacto-N-neotetraose (LNnT), can be manufactured by biofermentation and added to hypoallergenic formula. HMO are structurally and functionally different from other prebiotics, such as galacto-oligosaccharides (GOS) and fructo-oligosaccharides (FOS). GOS and FOS are also used to supplement infant formula but are not found in breast milk. The advantages of HMO, compared to GOS and FOS, are related to their greater structural and functional diversity of multiple components that act in synergy and confer protection to infants.
HMO-utilising infant-type bifidobacteria, which play a key role in early microbiome development, break down HMO to the molecular level and ferment them to produce lactate and acetate as their main metabolites.15 These in turn can be converted to other SCFAs, butyrate, and propionate by friendly cross-feeding bacteria.15 Heine explained that butyrate sits at the core of immune tolerance development in infants, which is highly relevant to CMA. It has two main target organs: the colon, where it acts to maintain mucosal integrity; and the immune system where it upregulates T-regulatory responses and downregulates inflammatory responses.16 Heine also introduced aromatic lactic acids as the “new kids on the block”. These immunomodulatory compounds are made by HMO-utilising infant-type bifidobacteria and have a potential role in early immune system development.17
Heine then moved on to consider lactose, which he described as a “neglected and misunderstood” compound in breast milk. Lactose can be utilised by infant gut bacteria as a substrate and acts to increase stool acidity, which is why breastfed infants typically have a lower stool pH of around 4.5–5. A whey-based extensively hydrolysed formula containing lactose has been shown to significantly increase bifidobacteria and lactobacilli, reaching counts similar to those found in healthy (breastfed) controls, while significantly reducing potentially pathogenic bacteria.18 Lactose also significantly increased concentrations of total SCFAs, especially acetate and butyrate.18 When lactose is combined with the two key HMO, 2’-FL and LNnT, it also demonstrates additive effects as illustrated by findings from a recent ex vivo faecal fermentation study (Figure 2).19 In that study of stool samples collected from 12 infants with probable CMA, HMO-utilising bifidobacteria and SCFA levels at 24 hours were increased after fermentation with HMO and/or lactose.19 In terms of safety, Heine confirmed that most manifestations of CMA can tolerate and benefit from lactose with the exception of cow’s milk protein-induced enteropathy.20 Cow’s milk-induced enteropathy may display a temporary lactose intolerance and lactose can generally be introduced again after a few weeks once diarrhoea has resolved.
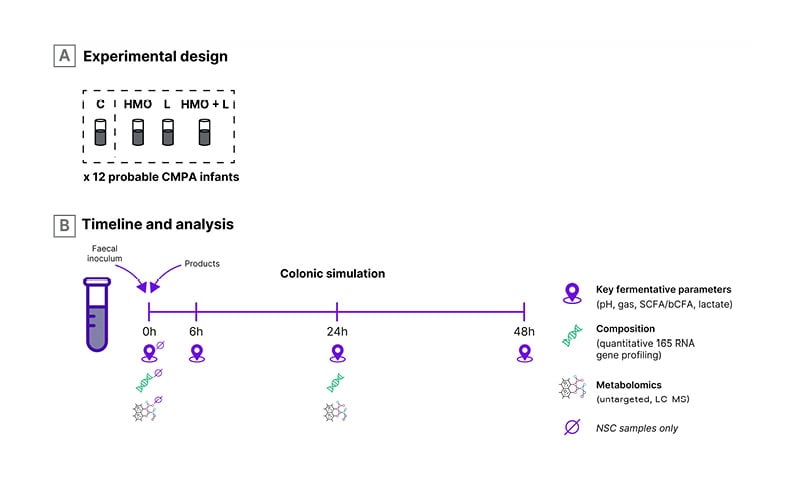
Figure 2: Ex vivo faecal fermentation study design.
bCFA: branched chain fatty acids; C: control without added substrate; CMPA: cow’s milk protein allergy;
2’-FL: 2’-fucosyllactose; HMO: human milk oligosaccharides; LC-MS: liquid chromatography-mass.spectrometry;
L: lactose; LNnT: lacto-N-neotetraose; NSC: non-supplemented control; SCFA: short chain fatty acids.
A clinical trial of a lactose-containing, whey-based extensively hydrolysed formula supplemented with 2 HMO, 2’-FL, and LNnT (Althéra HMO) showed a reduction in infection rates from enrolment to 12 months in infants with CMA with reductions of >30% in the incidence of lower respiratory tract infections, >70% in otitis media, and >40% in GI infections/acute diarrhoea.21 Evaluation of faecal community types from infants with CMA in this study showed that HMO-supplemented formula delayed the transition of the microbiome composition towards an adult-like pattern (i.e. faecal community type 5), which may prolong the window for early immune modulation and might be protective.22 Early enrichment of the four key types of infant-type bifidobacteria was also seen in the early enrolment cohort (age <3 months) of this study.22 In terms of metabolic signatures, faecal acetate levels increased in the HMO-supplemented group from baseline visit to 1-month follow-up and were reduced in control infants.22 Similar results were seen with an amino acid-based formula supplemented with 2’-FL and LNnT (Alfamino HMO), where there was significant enrichment of HMO-utilising infant-type bifidobacteria in infants with CMA from enrolment to 12 months of age.23 Collectively, these results illustrate that supplementation with 2’-FL and LNnT contributes to a healthier, age-appropriate gut microbiome, partially corrects dysbiosis, and may reduce infection rates in non-breastfed infants.
In summary, the infant microbiome plays a vital role in the development and management of allergic disease and is supported by lactose and HMO from breast milk. Supplementation of hypoallergenic formulas with breast milk-identical HMO (2’-FL and LNnT) was associated with immunological benefits, including a reduced rate of respiratory and GI infections in infants with CMA. The gut microbiome analysis from two studies in infants with CMA showed that HMO supplementation induced an early enrichment in HMO-utilising infant-type bifidobacteria and an increase in associated beneficial metabolites, such as acetate. Furthermore, ex vivo fermentation experiments suggest that the combination of lactose and HMO may confer additive effects on HMO-utilising infant-type bifidobacteria and faecal SCFA levels. The role of HMO supplementation on immunological tolerance development in infants with CMA and potential allergy prevention requires further clinical evaluation. “This is the next frontier,” said Heine, “to determine whether we can modulate the microbiome back into a healthier state and accelerate outgrowth from CMA.”
TIMINGS AND STRATEGIES FOR COW’S MILK REINTRODUCTION
Rosan Meyer
A wide variety of studies have explored the natural history of IgE-mediated CMA based on the OFC. In one recent study, persistent CMA was found to be associated with higher IgE levels, previous anaphylaxis, and complete cow’s milk elimination.24 This has led to the idea of whether earlier exposure to cow’s milk might help to speed up tolerance development.
Meyer described publications on the role of baked milk as a “bingo moment”. Across different studies, baked milk was shown to be tolerated by 68–76% of children with CMA.25-28 Importantly, subjects who incorporated dietary baked milk were 16 times more likely than the comparison group to become tolerant to unheated milk.25 Evidence for the tolerance of baked milk led to the birth of the milk ladder, various forms of which now exist but all with the proviso that they have been developed for non-IgE mediated CMA. However, as Meyer explained, clinicians are acutely aware of the use of milk ladders in IgE-mediated CMA, but there are concerns about safety and efficacy. To address this issue directly, a recent Irish randomised-controlled trial evaluated the use of milk ladder in infants with IgE-mediated CMA that are less than 12 months old.29 This age is critical, Meyer stressed, because it equates to maximum immune plasticity. Patients in the study were divided into two groups. Forty children (mean age: 7.3 months; mean skin prick test: 5.96 mm; IgE: 11.3 KUA/L) underwent a supervised ED05 challenge (i.e. were exposed to an eliciting dose of milk protein that is the dose expected to cause objective allergic symptoms in 5% of the milk allergic population), and those who passed then followed the 12-step milk ladder at home. The control group of 20 children (mean age: 7.9 months; mean skin prick test: 6.1 mm; mean IgE: 8.67 KUA/L) followed the standard practice of elimination and waiting for skin prick test/specific IgE size to come down. Results from this study (Table 1) showed that, by 6 months, significantly more patients in the early intervention group had reached Step 6 and Step 12 (fresh milk tolerance) of the milk ladder, compared to controls. By Month 12, the majority of these infants (64%) had achieved milk tolerance, significantly more than in the control group (37%).29
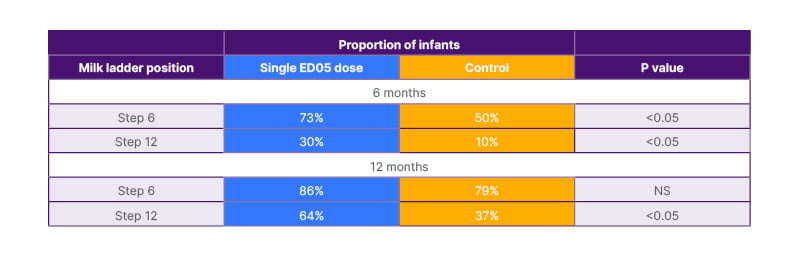
Table 1: Use of the milk-ladder in IgE-medicated cow’s milk allergy.29
ED05 is the dose expected to cause objective allergic symptoms in 5% of the milk-allergic population.
ED05: eliciting dose of milk protein; NS: not significant.
Meyer noted that the study did find mild reactions when transitioning to a higher step on the milk ladder. However, no child was given adrenaline at any time during this study and three had non-tolerated accidental exposures to milk to steps that were higher on the milk ladder than they tolerated.29 Nevertheless, she emphasised the need to exercise caution when using the milk ladder in IgE-mediated CMA and carefully select the patient population. This is particularly important as studies with the baked milk challenge have reported a 28% incidence of anaphylaxis in infants with this type of CMA.30
The World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) guidelines published in 2022 considered oral immunotherapies for CMA and concluded that this approach is a “balance” and “not a zero-risk procedure”, Meyer noted.31 Recommendations are therefore conditional due to the low certainty of the health effects based on evidence. However, in those infants where the benefit of having milk outweighs the side effects, milk oral immunotherapy can be considered.
In the area of non-IgE-mediated CMA, studies suggest that tolerance is achieved, and the allergy outgrown, earlier than in IgE-mediated CMA. However, less natural history data are available based on OFCs. Most guidelines suggest reintroduction to establish tolerance at least 6 months after the elimination diet has been started, but this is based on consensus rather than solid evidence.7,32,33 Meyer also highlighted confusion around the confirmation of CMA via a rechallenge, versus reintroduction of cow’s milk. For non-IgE mediated allergy one does not have a diagnosis until they have rechallenged for confirmation, she explained, usually after 2–4 weeks. The actual reintroduction, when tolerance is suspected, itself can then be carried out at home following the International Milk Allergy in Primary Care milk ladder.
Meyer acknowledged that there are both positives and negatives of the milk ladder approach. Multiple attempts may be required at some of the steps in order to achieve tolerance, as demonstrated in a recent study, so encouraging parents to persevere and retrial is important.34 In this respect, Meyer highlighted CoMiSSTM as a useful monitoring tool for cow’s milk reintroduction, as it provides an objective measure of symptoms. Finally, Meyer pointed to observational evidence suggesting tolerance of baked milk in children with food protein-induced enterocolitis syndrome (FPIES). Based on this, DRACMA guidelines now include a separate table setting out the preferred settings and pros/cons of using the milk ladder in this patient population, but more research is needed.33
Although the timing for reintroduction of cow’s milk is better established for IgE than non-IgE mediated allergies, the watch-and-wait approach from the past has now been replaced with a more active way of managing CMA, Meyer summarised. For IgE-mediated CMA, earlier intervention with baked milk and the use of the milk ladder approach has yielded promising results. For non-IgE mediated allergies, there is currently consensus about reintroduction at around 6–12 months of age (at least 6 months after elimination) but waiting until 1 year of age will not be required. The milk ladder approach is commonly used in non-IgE mediated allergies but is not routine practice for food protein-induced enterocolitis syndrome and eosinophilic oesophagitis. When using the milk ladder, Meyer reiterated the need to repeat steps as required and not just assume failure.
It is also critical to review patients to establish optimal timing on an individual basis. Parents’ compliance is key and guidance on cow’s milk reintroduction needs to be as clear as possible, Meyer concluded.
Q&A
All panel
The main symposium presentations were followed by a question and answer session in which all the expert panel members participated.
Staiano was asked for her perspectives on whether over or under-diagnosis of CMA posed more of a problem. She replied that, although the potential for over-diagnosis exists, CoMiSSTM constitutes a useful awareness tool for clinical practice, if interpreted correctly. Staiano added that, to diagnose CMA, involvement of at least two systems, such as GI and respiratory, is required, increasing the accuracy of CoMiSSTM.
A delegate in the audience asked Heine if there was any correlation between the increased number of HMOs and a further reduction in CMA risk. In the future, there will likely be an expansion of the spectrum of HMOs used in infant formula given the increasing technical feasibility of making these agents, he replied. He also added that it is worth noting that some non-CMA formulas now contain up to six HMOs. Diversification and an increase in the dose of HMO might lead to a greater impact on the microbiome, with related metabolic and health benefits, Heine suggested. However, studies will be needed to show if this reduces the rate of CMA or accelerates outgrowth.
Asked about the specifics of using CoMiSSTM in older infants, Staiano explained that the tool has already been validated for infants aged under 6 months. However, validation studies are ongoing in the 6–12-month age group, and results are expected by the end of the year.
A further question from the audience asked Heine about the practicalities of diagnosing dysbiosis. He acknowledged that this is not easy at the practical level, in the absence of advanced research tools such as deep sequencing. However, an infant stool pH of approximately 6 indicates a lack of bifidobacteria and SCFAs, which could be an indicator of dysbiosis. Moving forward, Heine highlighted the need for improved clinical tools in order to screen infants at an early age for the risk of non-communicable diseases and inflammatory disorders.
Meyer was asked if there is any evidence to support the length of time that should be left before rechallenging when a child fails a step of the milk ladder. She suggested that, given the lack of firm evidence, a pragmatic approach would be to rechallenge fairly rapidly if the failure was due to confounding factors, e.g. illness. However, if it was a clear failure, then depending on the age of the infant and the stage of the ladder, Meyer recommended waiting 6–8 weeks before rechallenging, especially if the infant scored highly on CoMiSSTM.
A final comment from the audience questioned whether non-IgE medicated CMA should be redefined as a condition related to cow’s milk protein, but not an allergy in terms of immunological disorders. Vandenplas acknowledged that it is often very difficult to differentiate between DGBI and non-IgE mediated CMA at the clinical level because there is no clear diagnostic test. From their perspective, the biggest weakness of thr gold standard is that a positive OFC does not indicate immune system involvement, it is simply a clinical reaction, he concluded.





