Meeting Summary
Prof O’Mahony began by discussing how the human gut is colonised by a wide diversity of microbes. He went on to review the evidence for how they exhibit highly evolved synergistic relationships to provide essential biological functions to the host and how the gut microbiome is influenced by many factors in early life. Prof Renz proceeded to describe the importance of establishing a stable gut microbial community, which closely tracks host growth and immune development. The mechanisms whereby delays or alterations in the establishment of these communities can lead to microbiome immaturity, raise the risk of allergy development including cow’s milk protein allergy (CMPA). Dr Forbes-Blom introduced the multiplicity of human milk oligosaccharides (HMO) and explained their position as multifunctional components that shape the developing gut microbiome and influence the developing immune system. Finally, Prof Nowak-Wegrzyn reviewed the latest trial data on supplementing specialty formulas for the management of CMPA with different HMO and reported the results.
The Development of the Gut Microbiome and its Role
Professor Liam O’Mahony
In the last few years, the human gut microbiome has become a very exciting area of research following the delineation that there are between 1013 and 1014 gut micro-organisms, with >1,000 species identified so far. This represents half of all cells in the human body, and, with 2–3 million genes, the microbiome substantially dwarfs the host genome which is estimated to have approximately 25,000 genes. The gut microbiome consists of bacteria, fungi, viruses, and bacteriophages. They secrete many primary and secondary metabolites which may be absorbed to influence multiple body sites. A third of all metabolites measured within human urine are bacterial in origin.
The host benefits for such a complex organ are extensive and can be categorised into four broad spheres of influence: i) these non-pathogenic bacteria displace pathogenic species; ii) many bacterial species are responsible for secreting a diverse range of molecules that stimulate the host immune system and drive immune tolerance; iii) gut bacteria impact nutrition and metabolism because they are responsible for generating additional nutrients which humans are incapable of producing from our ingested food as well as influencing our metabolism; iv) gut bacteria have been shown to influence behaviour and mood. These four spheres of influence can be far-reaching, and disruption of microbiome–host interactions have been implicated in diarrhoeal disorders, inflammatory bowel disease, allergies, asthma, autoimmunity, obesity, diabetes, and liver disease, as well as depression, anxiety, and attention deficit hypersensitivity disorder.
Development of an infant’s gut microbiome requires 1–2 years and is not bequeathed at birth but instead acquired over the first years of life. Koenig et al.1 completed an in-depth case study on the development of one infant’s gut microbiome over the first 2.5 years of life, showing how increasing abundance and diversity of bacterial species corresponded to shifts in diet and health events. To determine whether it is environmental or genetic influences that shape the gut microbiome, Rothschild et al.2 studied >1,000 adults with diverse genetic backgrounds and showed genetic kinship was only weakly associated with shaping their gut microbiomes and that it was non-genetic factors such as diet that had a much greater influence. Indeed, diet-bacterial interactions can result in beneficial metabolites; Brussow and Parkinson3 showed that, following consumption of indigestible plant fibre, bacteria resident in the gut can ferment these fibres to generate short chain fatty acids (SCFA) such as acetate, butyrate, and propionate. Propionate can directly initiate a gut–brain neural circuit which has beneficial effects on host physiology.
Roduit et al.4 examined 301 children from a birth cohort and measured SCFA levels in their faecal samples by high-performance liquid chromatography at 1 year of age. Associations with early life exposures, especially diet, allergy, and asthma later in their lives, were also examined. Children with the highest levels of faecal butyrate and propionate at the age of 1 year had significantly less atopic sensitisation and were less likely to have asthma by 6 years. They were also less likely to have a reported diagnosis of food allergy or allergic rhinitis.
Prof O’Mahony proposed the analogy of an infant’s gastro-intestinal tract being similar to a newly emerged island which undergoes a succession of colonisations as new species arrive and form communities, replete with extinction events when species are wiped out by environmental change or competition. Early life events, such as mode of delivery, breastfeeding, mother’s diet and health status, antibiotic and other drug usage in pregnancy and early childhood, the early-life environment such as presence of siblings, and the proximity to pets or farm animals, significantly affect the timing of bacterial colonisation and establishment, which can modify the risk of developing allergies and asthma.5 Additionally, humans have evolved over millions of years within an environmental and social context, which has facilitated a reliable transmission and dispersal of gut symbionts. Membership of the human microbiome metacommunity has also been driven by evolutionary factors and these human-adapted symbionts might only be acquired through contact with other humans. Modern lifestyles and changed social interactions may disrupt these metacommunity pathways which could lead to perturbations in human microbiomes. The consequences of this might be a cause for the observed increase in immune-mediated diseases. It is important that infants are given the opportunity to build up a full experience of such symbionts in early life.
The Role of the Gut Microbiome in Early Immune Development and Allergies
Professor Harald Renz
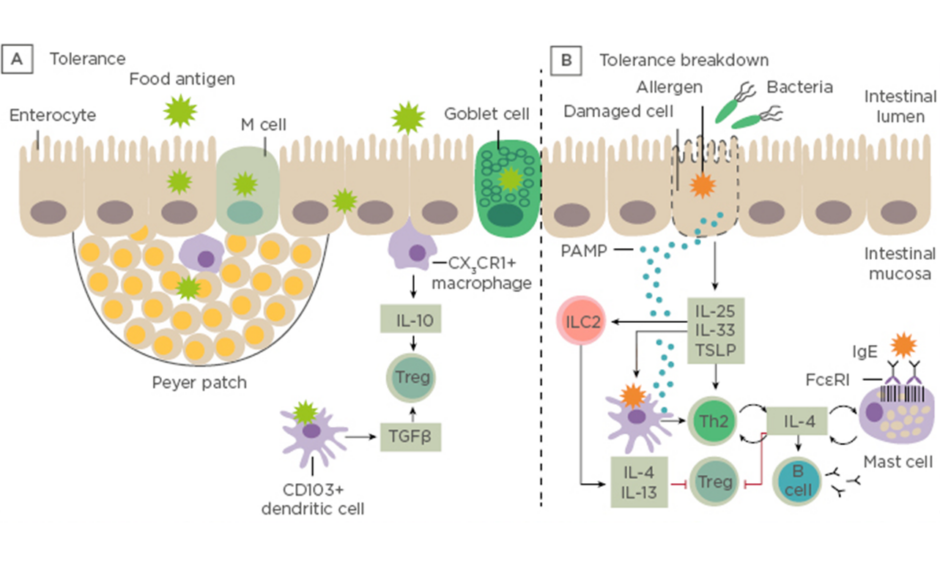
Prof Renz opened his talk with a short introduction to the gastrointestinal tract showing the difference between immune tolerance and breakdown of tolerance to ingested antigens (Figure 1).
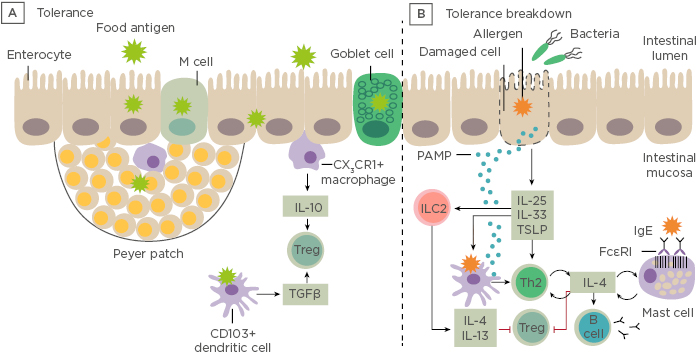
Figure 1: Immune tolerance and breakdown of tolerance to ingested antigens. A) Under normal conditions, food antigens in the gastrointestinal lumen pass into the intestinal mucosa by transiting between enterocytes or by active transport. B) Tolerance breaks down in situations in which danger signals arise. LC: innate lymphoid cells; PAMP: pathogen-associated molecular pattern molecules; Th; T helper cell; Treg: regulatory T cell; TSLP: thymic stromal lymphopoietin. Adapted from Renz et al., 20186
Dysbiosis in the gut microbiome can be defined by a qualitative and quantitative dysregulation of the composition of the microbiota indicating an impaired microbiome and can be observed preceding disease. A microbial exposome is defined as the measure of all the microbial exposures of an individual in a lifetime and how those exposures relate to health. The window of opportunity at the start of life enables the infant to acquire a library of environmental microbial encounters from the mother during pregnancy and birth, as well as intimate contacts from nasal, throat, milk, and skin encounters. Additionally, indoor pet encounters and microbial encounters outdoors and through ingestion of solid foods all contribute to this exposome. Feehley et al.7 have hypothesised that alterations in the microbiome interfere with immune system maturation, resulting in IgA production impairment, reduced regulatory T cell (Treg) abundance, and Th2-skewing of baseline immune responses to drive aberrant responses to innocuous (food) antigens.
Exposure to respiratory syncytial virus, rhinovirus, caesarean delivery, antibiotic use prenatally and in infancy, and infant obesity are all modifiable allergy and asthma risk factors contributing to the exposome. It has been shown that the development of the gut microbiome varies depending on the mode of birth delivery. Those born vaginally start with a Lactobacillus and Prevotella-dominated microbiome, while infants born through caesarean section have a Staphylococcus, Proprionibacterium, Streptococcus, and Corynebacterium-dominated microbiome. Sevelsted et al.8 investigated the link between caesarean delivery and the development of immune diseases such as asthma, allergy, inflammatory bowel disease, and Type 1 diabetes mellitus. There were 1.9 million full-term children born by caesarean delivery in the period from 1977–2011, who were analysed for chronic immune diseases as recorded in the Danish national registries. Children delivered by caesarean section had significantly increased risk of asthma, systemic connective tissue disorders, juvenile arthritis, inflammatory bowel disease, immune deficiencies, and leukaemia.
In a large population-based cohort study, Wu et al.9 reported that in utero and early-life events, including maternal urinary tract infections during pregnancy, maternal antibiotic use, caesarean delivery, infant antibiotic use, and having no older siblings at home were all associated with an increased risk of childhood asthma. In addition, there were strong dose-dependent relationships between a number of maternal urinary tract infections during pregnancy, maternal antibiotic use, infant antibiotic use, and older siblings at home with asthma risk. Individuals with extremes of multiple exposures had nearly 8-fold increased odds of developing asthma by the age of 6 years.
The modifiable protective factors in the developing microbiome were reviewed. Breast feeding, exposure to pets, and (in certain countries) the presence of Helicobacter pylori, have been shown to have beneficial effects. As the infant develops, the introduction of solid foods impacts the gut microbiome. During breast feeding, the gut microbiota is dominated by Bifidobacterium, Lactobacillus, and Veillonella. These microbes contribute to immune system development. As solid foods are started, so the taxa are seen to shift in favour of Bacteroides and Clostridiales which contribute to the development of SCFA. Roduit et al.,4 as reported by Prof O’Mahony, has shown how SCFA play a significant role in reducing atopic sensitisation, asthma, and food allergy at 6 years of age. The concept of the microbial exposome, in which high microbial burden confers inflammatory resilience, was discussed. McDade et al.10 had investigated whether patterns of DNA methylation in inflammatory genes in young adulthood would be predicted by exposure to the exposomes of early life nutritional, microbial, and psychosocial events. The pattern of results showed that repeated microbial exposure in infancy led to a trained immunity with strong resilience to chronic inflammation in adulthood, while low microbial exposure in infancy led to low resilience.
Dr Renz explained the importance of the ‘window of opportunity’: the critical period in life during which environmental factors exert a lasting effect on the individual and determine an individual’s susceptibility to developing allergies and certain lifestyle diseases in adult life. This period spans from intrauterine development through to at least the first 2 years in postnatal life, and is referred to as the ‘first 1,000 days’.11 By proactively managing their exposomes in this period, it is anticipated that children’s gut microbiomes will be optimised for future healthier lives.
What are Human Milk Oligosaccharides?
Doctor Elizabeth Forbes-Blom
A mother’s milk is the best nutrition for her infant and is associated with health benefits such as a lower risk of respiratory and gastrointestinal infections, obesity, diabetes, and possibly allergies.
Human breast milk contains many macro and micro-nutrients as well as HMO. Besides water content, lactose is the major constituent of breast milk with 70 g/L, followed by lipids at 40 g/L (Figure 2).
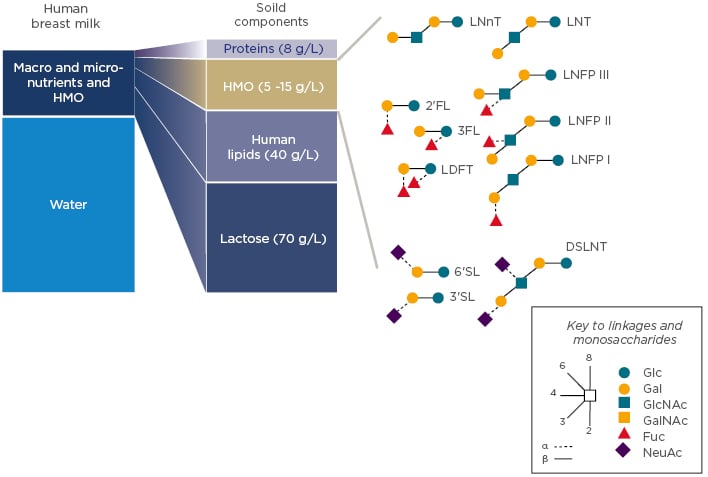
Figure 2: Gross composition of human milk with examples of major HMO structures. 2’FL: 2′-fucosyllactose; 3FL: 3fucosyllactose; 3’SL: 3’sialyllactose; 6’SL: 6-sialyllactose; DSLNT: disialyllacto-N-tetraose; Fuc: Fucose; Gal: galactose; GalNAc: N-Acetylgalactosamine; Glc: glucose; GlcNAc: N-acetyl-glucosamine; HMO: human milk oligosaccharides; LDFT: lactodifucotetraose; LNFP: lacto-N-fucopentaose; LNnT: lacto-N-neotetraose; LNT: Lacto-N-tetraose; NeuAc: sialic acid.
Human milk components can be categorised into those with nutritive value for the infant (lactose, proteins, and lipids) and those without nutritive value per se, as they are not digested and exert bioactivities; HMO are part of this second category. One of their main roles identified to date is to support the developing infant gut microbiome. HMO are structurally different from classical prebiotics such as galacto-oligosaccharides and fructo-oligosaccharides. Based on a lactose backbone, HMO are decorated and elongated with the monosaccharides galactose (Gal), N-acetyl-glucosamine (GlcNAc), fucose (Fuc), and sialic acid (NeuAc) with different linkage arrays (Figure 2). They resemble mucosal glycans at the host–microbe interface, and this molecular mimicry supports HMO to play important roles in orchestrating the host–microbial interactions via multiple mechanisms. These mechanisms include preventing pathogen growth and adhesion, reducing inflammatory responses and aiding the mucosal barrier function, as well as promoting an early life microbiome dominated by bifidobacteria. These data taken together demonstrate that HMO promote a gut ecosystem that is unfavourable for invading pathogens, known as colonisation resistance, and influence appropriate education of the developing immune system.
Proposed roles of HMO to protect against infections were explored as part of the secondary objectives in a randomised, placebo-controlled intervention trial by comparing infants fed a control formula and a formula supplemented with two HMO: 2’fucosyllactose (2’FL) and lacto-N-neotetraose (LNnT).12 The group of infants fed with the formula supplemented with HMO had a significantly lower risk to experience at least one reported respiratory tract infection and to require antibiotics during the first year of life.
Further investigations conducted by Berger et al.13,14 compared microbiota compositions at 3 months of age across two randomised formula-fed groups (formula supplemented with HMO and formula without HMO) and a breastfed reference group. The faecal microbiota composition could be categorised into three distinct faecal community types: A, B, or C. In the group that was fed formula supplemented with HMO and the breastfed reference group there was a higher percentage of infants with faecal community type B and a lower percentage of infants with faecal community type C, as compared to the group fed the control formula. Notably, formula fed infants with faecal community type C, typical of the control formula fed infants, had a hazard ratio of 2 (95% confidence interval [CI]: 1.1–3.9; p=0.02) to require antibiotics during the first year of life, as compared to formula-fed infants with faecal community type B that was typical for breastfed infants. Berger and Sprenger15 further segmented changes in the faecal microbiota composition of infants based on vaginal as compared to caesarean birth within each of the formula groups and the breastfed reference group. These results showed that feeding a formula supplemented with HMO reduced the risk for reported lower respiratory tract infections primarily in the caesarean section-born infants, together with positive changes in microbiota composition such as a strong increase in Bifidobacterium abundance.
Human Milk Oligosaccharides in the Dietary Management of Cow’s Milk Protein Allergy
Professor Anna Nowak-Wegrzyn
HMO come in different lengths and many act as nutrients for non-pathogenic bacteria such as Bifidobacterium infantis. Shorter chain HMO are almost exclusively consumed by these bacteria and are metabolised to produce SCFA. HMO have also been shown to restrict potential pathogens by providing decoy lectin-binding sites which mimic similar structures found in host epithelia.16
HMO are a complex mixture of bioactive components supporting the immune development of breastfed infants. Dendritic cells (DC) play a central role in the regulation of immune responses, being specialised in antigen presentation and driving T cell priming as well as differentiation. Xiao et al.17 reported on their elucidation of the effect HMO have on the maturation of the immune systems. They showed that a HMO mixture, isolated from pooled human milk, consistently induced semi-maturation of human monocyte-derived DC (moDC). HMO-conditioned human moDC promoted Treg generation from native CD4+ T cells. HMO contain tolerogenic factors that influence human moDC, and thereby modulate the development of the neonatal immune system.
Sprenger et al.18 studied HMO composition and genetic variation between women. HMO fucosylation is mediated by two fucosyl transferases: FUT2 and FUT3. Non-secretor mothers, who lack the functional FUT2 enzyme, also lack most α1-2-fucosylated oligosaccharides such as 2’FL and lacto-N-fucopentaose (LNFP). Infants fed by non-secretor mothers show a delay in establishing a bifidobacteria-laden microbiome. To compare the HMO composition in breast milk received by infants who develop CMPA with that in infants without CMPA, Seppo et al.19 classified infants into types of CMPA. They showed that all mothers of infants with delayed-onset CMPA were secretors (active FUT2, milk containing 29FL and LNFP I), while those with an infant with immediate-type (IgE-mediated) CMPA did not have active FUT2. Regardless of CMPA type, after correction for multiple comparisons, the level of LNFP III remained significantly lower in mothers with an infant with CMPA (29 mM versus 57 mM; 95% CI: 11–43; adjusted, p=0.0069). Infants who received low (<60 mM) LNFP III-containing milk were more likely to become affected with CMPA when compared with infants who received high LNFP III-containing milk (odds ratio: 6.7; 95% CI: 2.0–22.0).
There have been a series of studies carried out to evaluate the impact of adding HMO to infant formula. To establish whether infant formula with HMO is well tolerated by healthy infants, Storm et al.20 conducted a 6-week, randomised, controlled study of partially hydrolysed whey-based infant formula with 2’FL and Bifidobacterium lactis in healthy infants from 2 weeks of age. The control group received the same formula without HMO. Based on a gastrointestinal symptom questionnaire, outcomes were no different between groups. As already reported, the benefits of adding HMO to infant formula were studied by Puccio et al.,12 who found that at the end of the study the formula +HMO (2’FL and LNnT) group showed lower risk from infectious diseases and related medication use up to 12 months of age. A recent multicentre, randomised trial has been conducted to test hypoallergenicity of whey-based extensively hydrolysed formula (EHF) containing 2’FL and LNnT in IgE-mediated CMPA. Full term infants aged 2 months to 4 years, not breastfed, and with physician-confirmed IgE-mediated CMPA, were enrolled. The test formula group received the whey-based EHF Althéra (Nestlé Health Science, Vevey, Switzerland) with reduced content of extensively hydrolysed whey protein (2.2 g/100 kcal) with added 2’FL and LNnT, while the control group received Althéra with extensively hydrolysed whey protein (2.5 g/100 kcal) without added HMO. The criterion for formula hypoallergenicity is defined by the American Academy of Pediatrics (AAP): at a minimum, it must be confirmed that 90% of infants with documented CMPA will not react with defined symptoms to the formula under double-blind, placebo-controlled conditions. Results showed that >90% of subjects tolerated the novel whey-based EHF with two HMO, confirming its hypoallergenicity.21
Finally, Prof Nowak-Wegrzyn proposed hypotheses that still need further study: whether EHF with HMO has the potential to accelerate tolerance development in infants with CMPA by modulating the gastrointestinal microbiome and directly influencing Treg development and function. Additionally, whether EHF with HMO might reduce the frequency of infections and related drug use in infants and children with CMPA requires further study.