Chairperson: Alexandra F. Santos1-4
Speakers: Lars Bode,5,6 Anna Nowak-Wegrzyn,⁷ Sophie Nutten⁸
1. Department of Women and Children’s Health (Paediatric Allergy), School of Life Course Sciences, Faculty of Life Sciences and Medicine, King’s College London, London, UK
2. Peter Gorer Department of Immunobiology, School of Immunology and Microbial Sciences, King’s College London, London, UK
3. Children’s Allergy Service, Guy’s and St Thomas’ Hospital, London, UK
4. Asthma UK Centre in Allergic Mechanisms of Asthma, London, UK
5. Department of Pediatrics, University of California, San Diego, California, USA
6. Mother-Milk-Infant Center of Research Excellence, University of California, San Diego, California, USA
7. Allergy and Immunology, Department of Pediatrics, Hassenfeld Children’s Hospital, NYU Langone Health, New York City, New York, USA
8. Nestlé Health Science, Vevey, Switzerland
Disclosure: Prof Santos has received grants and personal fees from Medical Research Council (MR/M008517/1; MR/T032081); has received grants from Asthma UK and the NIHR through the Biomedical Research Centre (BRC) award to Guy’s and St Thomas’ NHS Foundation Trust, during the conduct of the study; grants from Immune Tolerance Network/National Institute of Allergy and Infectious Diseases (NIAID, NIH) and Asthma UK; has received personal fees from Thermo Scientific, Nutricia, Infomed, Novartis, Allergy Therapeutics, Buhlmann, and Stallergenes; and has received research support from Buhlmann and Thermo Scientific through a collaboration agreement with King’s College London. Prof Bode has received travel reimbursements from universities, foundations, and societies to speak at national and international grand rounds and conferences; and his research in the Bode laboratory and the University of California San Diego Mother-Milk-Infant Center of Research Excellence (MOMI CORE) is currently supported by the National Institutes of Health (NIH), National Science Foundation (NSF), Bill & Melinda Gates Foundation (BMGF), and Family Larsson-Rosenquist Foundation (FLRF). Prof Nowak-Wegrzyn has received honorarium for this presentation; and is an investigator for a clinical trial evaluating hypoallergenicity of a hypoallergenic infant formula with human milk oligosaccharides. Dr Nutten is an employee of Nestlé Health Science.
Acknowledgements: Writing assistance was provided by Satyendra Shenoy, Describe Scientific Writing & Communications, Cologne, Germany.
Support: The symposium and the publication of this article was funded by Nestlé Health Science, Vevey, Switzerland.
Citation: EMJ Allergy Immunol. 2020;5[Suppl 2]:2-10.
Meeting Summary
This symposium, which was part of the industry programme at Food Allergy and Anaphylaxis Meeting and the European Consortium on Application of Flow Cytometry in Allergy (FAAM-EUROBAT) Digital 2020, was designed to provide an update on recent advances in our understanding of the role of human milk oligosaccharides (HMO) in the management of infant food allergies, specifically cow’s milk protein allergy (CMPA). The symposium was introduced by Prof Santos with a short definition of HMO and an overview of their role in orchestrating host–microbial interactions via multiple mechanisms; for instance, preventing pathogen growth and adhesion, reducing inflammatory responses, and aiding the mucosal barrier function, as well as promoting a beneficial microbiome in early life. Prof Bode outlined the key research into the role of HMO in an infant’s overall health. HMO have several physiological functions, direct and indirect, in the development of infant immune function. Hence, these functions could be utilised for the management of certain food allergies, such as CMPA. Prof Nowak-Wegrzyn discussed recent developments in the management of CMPA in infants, specifically relating to two key HMO: 2’-fucosyllactose (2’-FL) and lacto-N-neotetraose (LNnT). Prof Nowak-Wegrzyn highlighted the importance of the microbiome in the development of the infant’s immune system and described the results of recent clinical trials that demonstrated the safety and beneficial effects of formula supplemented with 2’-FL and LNnT in healthy infants and those with CMPA. Briefly, infants receiving formula supplemented with HMO had a lower incidence of respiratory tract and other infections and hence a reduction in exposure to medications, such as antibiotics and antipyretics. Dr Nutten explained how current knowledge could help explore the role of HMO in food allergy and sensitisation. Towards this end, Dr Nutten highlighted the importance of HMO, individually and collectively, in maintaining conditions optimal for infant health, and shared data from studies aimed at unravelling the mechanisms through which HMO affect the infant gut microbiome.
Human Milk Oligosaccharides for Immune System Development
Professor Lars Bode
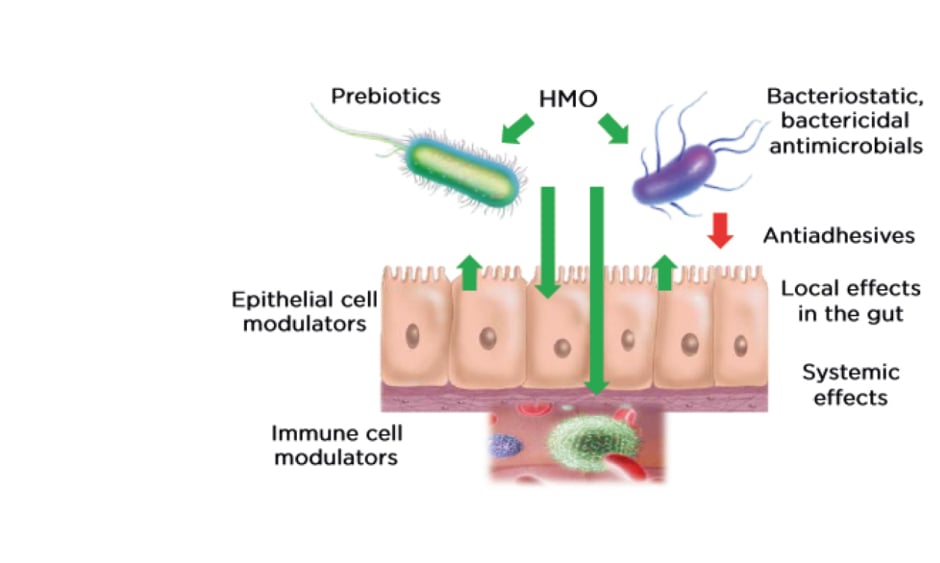
Breast milk is the gold standard in infant nutrition and plays a key role in infant survival and health. According to the editorial from The Lancet’s 2016 series on breastfeeding, “The deaths of 823,000 children and 20,000 mothers each year could be averted through universal breastfeeding, along with economic savings of US$300 billion.”1 Hence, research on the constituents of breast milk, as well as their mechanisms, could lead to better understanding of the role of breast milk in improving infant health. HMO are the third largest nonaqueous component of breast milk, after lactose and fat, and are present in concentrations of 5–15 g/L. Bovine milk, in comparison, contains approximately 100- to 1,000-fold lower concentration of oligosaccharides. HMO are complex sugars derived from combinations of five monosaccharides: glucose, galactose, N-acetylglucosamine, fucose, and sialic acid. The physiological functions of HMO depend on the final structure of the macromolecule resulting from the combination of these building blocks, as well as further chemical modifications, such as fucosylation or sialylation. Over the years, 150–200 distinct HMO have been identified. The expression of individual HMO varies from mother to mother, and results of the Canadian CHILD study cohort of 1,200 mothers have revealed a large heterogeneity in the absolute concentration and relative abundance of HMO in breast milk, which is dependent on multiple intrinsic and extrinsic factors.2 Breast milk composition changes over the course of lactation in an individual mother, but intraindividual HMO levels are remarkably constant over short periods of time, i.e., within 1 week or within 1 day, although at the interindividual level, composition varies from mother to mother. HMO, once ingested, pass through the gut largely unchanged owing to their resistance to low pH and pancreatic and brush border enzymes. Approximately 1% are absorbed in the small intestine and appear in the urine, while 10–80% are excreted via faeces following bacterial degradation in the colon.3 HMO play numerous roles in the infant gut (Figure 1). They serve as prebiotics: a food source for beneficial bacteria residing in the gut. In addition, HMO are protective against certain harmful pathogens owing to their antimicrobial properties and by preventing bacterial adhesion to the intestinal epithelium. HMO also have direct effects on the epithelial and immune cells, which may subsequently affect the gut microbial composition. Despite this growing body of research, there is still a vast amount of knowledge yet to be discovered regarding the role of HMO. Various approaches ranging from in vitro and ex vivo studies to in silico modelling to human cohort studies and clinical trials are being employed worldwide in research on HMO.
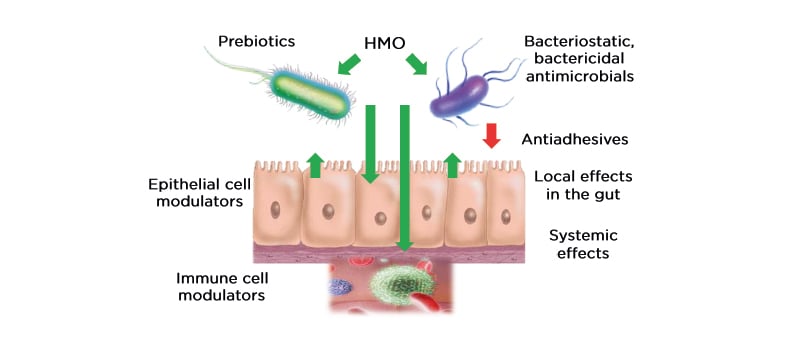
Figure 1: The role of human milk oligosaccharides in infant health. HMO not only serve as food source for beneficial bacteria, but they also possess antimicrobial and antiadhesive properties that prevent growth and proliferation of harmful bacteria. HMO also have systemic effects on epithelial and immune cells which further assist in maintenance of a healthy microbiome. HMO: human milk oligosaccharides.
The specific effects of individual HMO have already been demonstrated in in vitro and animal studies: the antimicrobial/antiadhesive effects of 2’-FL, lacto-N-tetraose, 3’-sialyllactose (3’-SL), and 6′-sialyllactose (6’-SL);4-6 the effects of 2’-FL, 3’-SL, 6’-SL, and LNnT on infant growth7-10 and neurodevelopment;11-14 and the effects of 2’-FL, 3’-SL, and 6’-SL on immune development in infants.15-19 Numerous human cohort studies have also revealed noteworthy associations between HMO and infant health. Seppo et al.20 showed that infants who received breast milk with low levels of the HMO lacto-N-fucopentaose III (LNFP-III) were more likely to develop CMPA. The CHILD study has also shown that specific HMO profiles are associated with food sensitisation in infants.21 These findings are consistent with findings from a larger cohort who were followed for 18 years to investigate the association between HMO profiles of breast milk and development of allergies; it was not only individual HMO, but also groups and clusters of HMO that were predictive of allergic disease.22 Finally, Prof Bode cited two recent review articles by Triantis et al.23 and Doherty et al.,24 which summarised the benefits of HMO in immune-mediated disease and infections in infancy.
In conclusion, HMO play a crucial role in infant health and current trends in HMO research hold the potential of alleviating immunologic conditions in infants who cannot be fed breast milk.
Benefits of Human Milk Oligosaccharides in the Management of Cow’s Milk Protein Allergy
Professor Anna Nowak-Wegrzyn
The gut microbiome in infants plays a key role in immune maturation and immunoregulation by modulating effector or tolerant responses to different antigens and balancing the activities of Th1 and Th2 immune cells.25 During infancy, the predominant beneficial bacteria in the gut are Bifidobacterium and lactobacilli;26 their metabolites, specifically short-chain fatty acids (SCFA), influence the development of the infant immune system through a variety of physiological processes. In breastfed infants, breast milk influences gut microbiome diversity and promotes higher concentrations of Bifidobacterium and lactobacilli. In addition, certain constituents of breast milk (secretory IgA, HMO, lactoferrin, and antimicrobial peptides) help the infant’s immune system by acting as barriers to pathogenic bacteria in the gut or providing indirect immune support. Lactose in breast milk has been shown to upregulate defences, especially against gut infections, through antimicrobial peptides.27 Additionally, HMO act as substrates for Bifidobacterium, driving up levels of SCFA.
In comparison to infants without food allergies, the gut microbiome of infants with food allergies reportedly has lower levels of Bifidobacterium and lactobacilli, and higher levels of Clostridium, staphylococci, and Escherichia coli. When breastfeeding is not possible for any reason, extensively hydrolysed formula (EHF) with lactose should be used as the first-line choice in infants with CMPA. Lactose supplementation has been shown to result in an increase in bacterial counts for Bifidobacterium and lactobacilli (p<0.01) and a concomitant decrease in the counts of harmful bacteria, such as Bacteroides and clostridia (p<0.05), in infants with CMPA.28 Moreover, the investigators reported an increase in concentrations of SCFA (p<0.03), especially acetate and butyrate, confirming the ability of lactose supplementation to restore a healthy gut microbiota. Of the SCFA, butyrate plays a prominent role in maintenance of intestinal epithelial and enterochromaffin cell integrity, as an energy source for colonocytes, mucin release, absorption of water, anti-inflammatory effects, interaction with T-regulatory (Treg) cells, and barrier function of the gastrointestinal tract. Correspondingly, low faecal butyrate levels have been reported in allergic infants aged up to 1 year.29 Other factors known to be associated with infant allergies are low IgA levels, early antibiotic exposure, caesarean birth, microbial dysbiosis and immune disturbances, and impaired intestinal permeability. The effects of an atypical gut microbiome on predisposition to allergy commences as early as the neonatal stage. In an investigation of a birth cohort, it was reported that the group with the highest microbial diversity, including lower relative abundance of certain bacteria such as Bifidobacterium, Akkermansia, and Faecalibacterium and a higher concentration of specific IgE at 2 years, had the highest risk of developing allergies.30 Hence, neonatal gut microbiota influence susceptibility to childhood allergies via alterations in the gut microenvironment. Lower respiratory tract infections (LRTI) are common in infants and young children aged 1–3 years and contribute significantly to morbidity and mortality, healthcare resource use, and quality of life.31 Infants with CMPA have a higher propensity for developing infections, such as LRTI, with a subsequent higher use of antibiotics, which is itself a risk factor for allergies.32
Prof Nowak-Wegrzyn then focussed on the specific role of two HMO, 2’-FL and LNnT, in infants with CMPA. These neutral oligosaccharides are the most abundant HMO in breast milk, accounting for approximately 30% of the total HMO content.33-36 The individual concentrations of these HMO is highest in the immediate post-partum period, which indicates a role in the development of the neonatal gut microbiota.33 Trials of infant formulas supplemented with 2’-FL and LNnT conducted in healthy infants showed that infants had a lower incidence of LRTI, bronchitis, and antibiotic usage in comparison with infants who received formula without HMO (relative risk for bronchitis: 0.37; 95% confidence interval [CI]: 0.18–0.75, relative risk for antibiotic use: 0.69; 95% CI: 0.51–0.92).37 These findings indicate that HMO are protective against dysbiosis. Another study investigating the hypoallergenicity of a whey-based EHF (w-EHF), supplemented with 1.0 g/L of 2’-FL and 0.5 g/L of LNnT (Althéra® HMO, Nestlé Health Science, Vevey, Switzerland), confirmed that >98% of the infants and young children aged <4 years with CMPA tolerated the supplemented w-EHF in both the modified intent-to-treat cohort and the per-protocol cohort.38 Thus, the w-EHF with additional 2’-FL and LNnT meets the clinical criteria for hypoallergenicity set by the American Academy of Pediatrics (AAP) and can be recommended for the management of CMPA in infants and young children. Results from the international, multicentre CINNAMON trial investigating the effect of the w-EHF supplemented with 1.0 g/L of 2’-FL and 0.5 g/L of LNnT with a lowered protein content, on infants with CMPA showed that there was no difference in growth using weight-for-age and length-for-age z-scores at 4 months post-baseline between supplemented and control formula.39 An interesting finding from this trial was the observed reduction in relative risk for infections (LRTI, upper RTI, otitis media, and gastrointestinal tract infection), as well as related medication (antibiotics and antipyretics) usage. Moreover, there was a reduction in the mean number of infectious episodes for LRTI (p= not significant) and upper RTI (p=0.003) in infants receiving the w-EHF supplemented with 2’-FL and LNnT. Prof Nowak-Wegrzyn also shared further secondary data from the CINNAMON trial (presented at FAAM-EUROBAT Digital 2020) that showed, for the first time, the existence of a lower α-diversity in the gut microbiota of infants who received the w-EHF supplemented with 2’-FL and LNnT (Figure 2).40 Although it is assumed that diversity is beneficial for the development of infant gut microbiota, healthy breastfed infants are found to have lower diversity, with gut microbiota dominated by bifidobacteria and lactobacilli. Pedersen et al.40 reported that microbial richness and Shannon index (a measure of microbiome diversity) were noticeably lower in infants with CMPA who had received the w-EHF supplemented with 2’-FL and LNnT, which reached a statistical significance by 12 months of age (p<0.001).
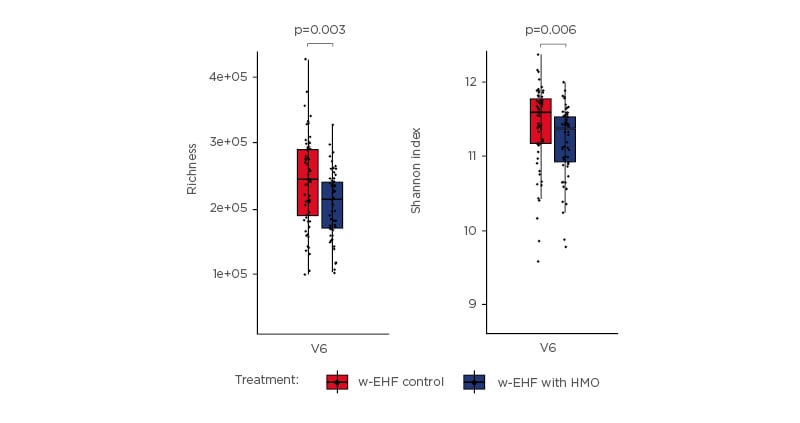
Figure 2: At 12 months, infants who received whey-based extensively hydrolysed formula supplemented with 2’-fucosyllactose and lacto-N-neotetraose had a lower α-diversity. Differences in gene richness and Shannon diversity between the gut microbiomes of infants receiving either the control formula or test formula, stratified by visit. Groups were compared pairwise by Mann–Whitney U test.40 w-EHF: whey-based extensively hydrolysed formula; HMO: human milk oligosaccharides. Adapted from Pedersen et al.40
Moreover, the shift of microbial clusters from ‘early type’ to ‘late type’ gut microbiome, which is typically seen in infants with CMPA around 1 year of age, appeared to have been restored to the early type with more beneficial bacteria, such as Actinobacteria, in infants fed the w-EHF supplemented with 2’-FL and LNnT (p=0.014).
Prof Nowak-Wegrzyn concluded by emphasising the role played by HMO in rectifying the dysregulation of gut microbiome in infants predisposed to allergic disease. w-EHF containing 2’-FL and LNnT (Althéra HMO) has been shown to be safe and well tolerated in infants with CMPA and appears to be protective against several infections.
Moreover, w-EHF supplemented with 2’-FL and LNnT appeared to delay the premature shift in gut microbiota to the adult type, a phenomenon indicative of future allergy development in infants who receive little or no breastfeeding.
Impact of Human Milk Oligosaccharides in Food Allergy
Doctor Sophie Nutten
Although breast milk is recommended for prevention of allergic diseases in high-risk infants, the exact underlying mechanisms are yet to be completely elucidated. Breastfeeding has been inconsistently associated with allergic conditions,41 reflecting the differences in breast milk constituents in different settings and populations. Breast milk contains various factors that affect immune development in the neonatal gut and could have an impact on allergic diseases. HMO are one of the most extensively studied major bioactive milk components. While devoid of a particularly nutritive value, HMO have numerous functions, including maintenance of the infant gut microbiome and development of infant immune system. Given these findings, it is suggested that HMO could also have a protective role in the development of allergies.
Dr Nutten shared data from recent observational studies. A Finnish observational study that investigated the role of fucosyltransferase 2 (FUT2)-dependent HMO in allergic disease later in life showed a lower incidence of IgE-associated allergies and eczema at age 2 years in infants delivered by caesarean section who were fed breast milk containing FUT2-dependent HMO (p<0.1).35 The expression of 2’-FL, one of the HMO regulated by the FUT2 gene, varies substantially in mothers with FUT2 polymorphism and hence the investigators probed further for an association between 2’-FL expression and allergic manifestation. Of note, the incidence of IgE-associated allergies and eczema in the caesarean-born infants was inversely proportional to the level of 2’-FL in the breast milk received during breastfeeding. At 2 years, a lower incidence of IgE-associated allergies and eczema was seen in caesarean-born infants fed breast milk containing FUT2-dependent HMO (Figure 3).
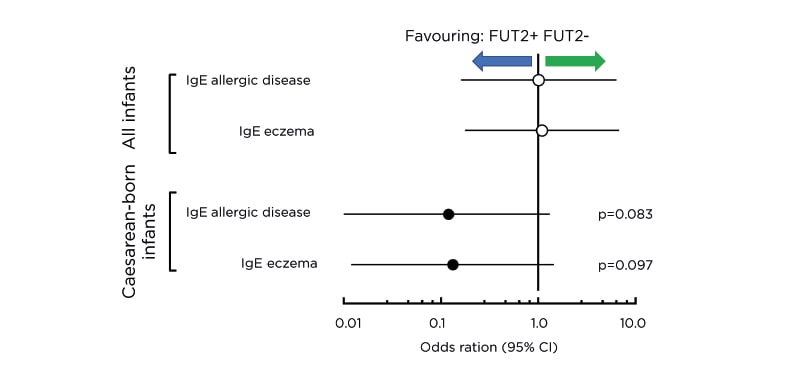
Figure 3: Fucosyltransferase 2-dependent human milk oligosaccharides and allergic disease. Risk of IgE-associated allergic disease and IgE-associated eczema at 2 years of age in caesarean-born infants who consumed FUT2-positive breast milk was substantially lower than those who consumed FUT2-negative breast milk. CI: confidence interval; FUT2: fucosyltransferase 2.
In the aforementioned study by Seppo et al.,20 besides LNFP-III, the investigators also noted substantially lower levels of other HMO (disialyllacto-N-tetraose, 6’SL, and LNFP-I) in the breast milk of mothers whose infants were at a higher risk of CMPA. Despite these findings, it is unclear whether individual HMO are always implicated in the development of allergies. An analysis of a Canadian cohort that investigated the role of 19 different HMO could not identify any association between these individual HMO and food sensitisation.21 However, the investigators noticed a difference in pattern of HMO composition between infants with and without food sensitisation: infants who received breast milk with higher levels of six HMO and lower levels of four HMO were associated with food sensitisation in the first year of life. Another recently published report on a long-term Australian study in a high allergy risk birth cohort found that exposure to acidic Lewis HMO profiles was associated with a higher risk of allergic disease and asthma (odds ratio: 5.82; 95% CI: 1.59–21.23), while exposure to breast milk with an acidic-predominant HMO profile was associated with reduced food sensitisation (odds ratio: 0.08; 95% CI: 0.01–0.67).22 This leads to the conclusion that it is not only levels of individual HMO but also the overall composition profile of HMO that influence development of allergies. Dr Nutten also shared unpublished data from a German cohort in which the investigators observed a nonlinear association between specific levels of 2’-FL and LNnT and incidence of infant allergy.
Certain FUT3 polymorphisms are known to result in increased levels of 2’-FL and this could perhaps explain the nonlinear association; however, further studies are required to establish causality.
Having established a case for the role of HMO composition in the development of food allergies and its implication on infant nutrition, Dr Nutten proceeded to discuss the results of preclinical studies that investigated the role of HMO in animal models of allergies. In an ovalbumin-sensitised mouse model of food allergy, supplementation with the HMO 2’-FL or 6’-SL could decrease the symptoms of food allergy, measured by diarrhoea score, in mice.42 Furthermore, the investigators showed an increase in Treg cells, as well as an indirect stabilisation of mast cells, which are largely responsible for allergic response in the mouse intestine.
This observation established mechanisms through which HMO supplementation resulted in the decrease of the treated animal’s symptoms. In another recent study, supplementation with 3’-SL in two different mouse models of atopic dermatitis was shown to result in the protection against allergic sensitisation (decrease in IgE production in a dose-dependent manner) and symptoms associated with a decrease in mast cell numbers.43 These data highlight the effectiveness of HMO in modulating the immune response at the whole animal level.
Finally, Dr Nutten shared unpublished data from in vitro mechanistic studies on 2’-FL and LNnT against food allergy. In a cellular model using peripheral blood mononuclear cells, treatment with a mixture of 2’-FL and LNnT resulted in a decrease in the production of IL-5, one of the key modulators of allergic disease. Moreover, incubation of mast cells with the 2’-FL–LNnT mixture resulted in a decreased degranulation of the former, indicating that 2’-FL/LNnT stabilised mast cells at tested concentrations. To investigate the effects of 2’-FL and LNnT on the gut epithelial barrier, the investigators preincubated intestinal epithelial cell lines, CaCo-2 and HT29-MTX treated with TNFα and IFNγ to mimic inflammatory conditions, with 2’-FL and LNnT. Pretreated cellular monolayers were found to have a significantly lower permeability, measured by the FITC-dextran-4 flux, in comparison with untreated cells. Progressing to animal studies, in the ovalbumin-choleratoxin mucosal sensitisation mouse model, supplementation of the animals’ diet with 2’-FL and LNnT at different concentrations reduced sensitisation and increased Treg counts in the animals. Moreover, a decrease in mast cell numbers and mast cell degranulation was observed in animals who received supplementation. Similar results were noticed in the Aspergillosis fumigatus-induced epicutaneous sensitisation mouse model; animals with diets supplemented with 2’-FL and LNnT displayed reduced sensitisation compared with control animals. Moreover, the investigators noted a dose-dependent effect of supplementation on gut microbiota composition, the highest difference reaching up to 10%.
In conclusion, both human and preclinical data are promising. Yet, further investigation on the potential of HMO supplementation for allergy management in infancy is warranted.