Meeting Summary
During this symposium, leading experts in paediatric allergy and immunology examined evidence for the immunomodulatory role of human milk oligosaccharides (HMO) in infants with cow’s milk protein allergy (CMPA), and considered the implications for clinical management. Mechanisms underpinning the positive modulatory effect of HMOs on the early microbiome and immune system responses in healthy infants and those with CMPA were explored by Liam O’Mahony, University College Cork, Republic of Ireland. Anna Nowak-Węgrzyn, Professor of Pediatrics at the New York University (NYU) Grossman School of Medicine, and Chief of the Division of Pediatric Allergy and Immunology at Hassenfeld Children’s Hospital, NYU Langone Health, New York City, USA, then reviewed findings from the CINNAMON and PLATYPUS clinical trials, highlighting the beneficial impact of HMO-supplemented formula on the clinical management of infants with CMPA. In these studies, hypoallergenic formulae containing the two key HMOs, 2′-Fucosyllactose (2’-FL) and Lacto-N-neotetraose (LNnT), showed a good safety profile, supported normal infant growth, and, importantly, helped to reduce the risk of respiratory infection in children with mild, moderate, or severe CMPA. In the final presentation of the symposium, Ralf Heine, Global Medical Director of Paediatric Care at Nestlé Health Science, Switzerland, showcased new data from the CINNAMON and PLATYPUS studies, shedding further light on the mechanisms by which HMOs can shape the early microbiome and influence the metabolome profile associated with important immune benefits in CMPA.
![]()
Corrigendum: How Do Human Milk Oligosaccharides Modulate the Immune System in Infants with Cow’s Milk Protein Allergy? Emerging Evidence and Clinical Implications
Chairpeople: Alexandra Santos
Speakers: Liam O’Mahony, Anna Nowak-Węgrzyn, Ralf G. Heine
Original citation: EMJ Allergy Immunol. 2023;8[1]:22-31. DOI/10.33590/emjallergyimmunol/10306540. https://doi.org/10.33590/emjallergyimmunol/10306540.
Date correction published: 21.07.23
The article funded by Nestlé Health Science in EMJ Allergy & Immunology 8.1 (pp.22–31) was originally published on 20.07.23. The abbreviation “faecal community type (FCT5)” on page 27 originally read “fibronectin, collagen, T-antigen type 5 (FCT5)” incorrectly. This has now been updated. In the key to Figure 2, “FCT5: faecal community type 5” also originally read “FCT5: fibronectin, collagen, T-antigen type 5” incorrectly. This has now been updated.
EMJ apologises for the error and any inconvenience caused.
![]()
Mechanisms of Human Milk Oligosaccharides in Modulating Immune Responses
Liam O’Mahony
Human mucosal surfaces and body cavities harbour diverse communities of commensal microbes that play essential roles in the regulation of host metabolic responses, epithelial barrier function, immune education and immunoregulation.1 From a sterile beginning, infants acquire these key microbes via many routes, including maternal transmission, from older siblings, and via contact with the wider environment.2 However, in the modern world, development of the early life microbiota can be hampered by factors such as Caesarean section delivery and antibiotic use, as well as smaller households and social network sizes.2 Once microbes are acquired, they must also be supported by the diet, explained O’Mahony, with dietary elements such as HMOs playing a particularly vital role in early-life microbiome development.
The microbiome develops in concert with the early-life immune system, with both the composition of the microbiome and its metabolic outputs helping to co-ordinate and drive communication pathways between early immune cells. This enables effector and regulatory networks to be established within the immature immune system, the failure of which can result in allergy.
To illustrate the importance of diet for early-life microbiome development, O’Mahony presented results from an unpublished study showing that 20–25% of microbiome variance in children aged 6–12 months can be explained by diet alone, an impact far greater than Caesarean section delivery. The main standout dietary factor contributing to a varied microbiome development in this study was breastfeeding. HMOs are one of the most important and abundant components of human breast milk, and over 150 different types have been identified. These complex sugars cannot be utilised by the human host, but instead are designed to support the growth and metabolism of protective commensal microbes, particularly infant-type bifidobacteria.
Many factors influence the HMO composition of a mother’s breast milk, and the ensuing differences can have an important impact on outcomes, such as food allergy. Studies have shown that relative HMO levels in breast milk correlate with CMPA risk in offspring, mediated via HMO effects on the immune system.3,4 The positive effect of HMOs also extends to infection risk, with supplementation of infant formula with the two key HMOs, 2’-FL and LNnT, shown to reduce the early-life incidence of bronchitis, lower respiratory tract infection, and antibiotic/antipyretic usage in otherwise healthy infants.5
Looking at the mechanisms by which HMOs act to positively modify allergy and infection risk, O’Mahony explained that HMOs exert both direct and indirect effects on the immune system. Indirect immune modulatory mechanisms include the expansion of immunoregulatory protective species, such as bifidobacteria, in the early gut microbiome.
Bifidobacterial strains have been shown to be important inducers of T regulatory cells in both mouse model experiments and human studies, an effect mediated via extracellular polysaccharides.6-8 In animal studies, removal of these key immunomodulatory cell structures was associated with significant increases in levels of inflammatory mediators, such as TNF-α and eosinophils.9,10 Bifidobacteria supported by dietary HMOs also play an important role in protection against infection. In a lethal influenza model in mice, just one intranasal application of bifidobacteria cell wall conferred a 50–60% increase in survival rates, accompanied by a significant reduction in viral titres versus placebo-treated animals.11
In addition to supporting the growth and metabolism of bifidobacteria, HMOs also act to modify microbial metabolism and generate key immune regulatory compounds in the early-life microbiome. As a result, infant metabolomic profiles are seen to change dramatically with HMO supplementation.12 Important metabolites include the short-chain fatty acids (SCFA) acetate, propionate, and butyrate, which engage with host cell receptors, and also influence the immune system epigenetically via histone deacetylase inhibition.13 Collectively, these effects induce an immune regulatory response, and protect against allergic sensitisation and viral respiratory tract infection. Higher levels of SCFAs in early life have been linked to a lower rate of sensitisation to both inhalant and food allergens at 6 years of age.14 In terms of disease outcomes, children with higher levels of butyrate also displayed a lower prevalence of asthma, allergic rhinitis, and food allergy by the age of 6 years.14
Finally, HMOs also exert direct actions on epithelial cells and immune cell subsets within the mucosa. These effects include the prevention of pathogen adhesion and infection, modification of innate immune cell activity, and improvement of epithelial barrier function.
Human breast milk contains many immunomodulatory factors, of which HMOs are undoubtedly one of the most important, concluded O’Mahony. HMO effects can be direct or indirect, and help to steer the development of vital regulatory and effector networks within the early-life immune system.
Clinical Implications of 2′-Fucosyllactose and Lacto-N-neotetraose in the Nutritional Management of Cow’s Milk Protein Allergy
Anna Nowak-Węgrzyn
CMPA currently ranks as the most prevalent food allergy in young children, with an incidence of 2–3% in the first year of life.15 It is an immune-mediated disease characterised by immune system hyperactivity, increased gut permeability, and altered gut microbiota, giving rise to intestinal dysbiosis.16-19
Nowak-Węgrzyn explained that infants and children with CMPA have a higher risk of developing infectious complications, predominately lower respiratory tract infections (LRTI), which is reflective of the T-helper (Th)1/Th2 imbalance and delayed immune system maturation.20,21 The control and elimination of intracellular pathogens requires the activation of immune responses, which are often hampered in CMPA, thereby increasing susceptibility to these infections.22 More infections equate to more oral antibiotics, Nowak-Węgrzyn continued, with those who have CMPA receiving an average of three courses in their first 3 years of life.21
HMOs, notably 2’-FL and LNnT, which are the most abundant in human breast milk, play a key role in supporting the infant immune system in CMPA. They promote beneficial bacteria, thereby protecting from dysbiosis associated with CMPA, and also boost gut barrier function to counteract CMPA-associated increased permeability.17,18,23-26 In addition, HMOs block pathogen adhesion in the gut to protect against respiratory tract infections, and directly modulate the immune system, helping to rebalance the abnormal Th1/Th2 responses seen in CMPA.17-20,23-25,27,28
A new generation of hypoallergenic formula incorporates the two key HMOs, 2’-FL and LNnT, which are identical to those found in maternal breast milk. The hypoallergenicity of the whey-based, lactose-containing, extensively hydrolysed formula (w-eHF), supplemented with 2’-FL and LNnT (Althéra® HMO, Nestlé Health Sciences, Vevey, Switzerland) was confirmed in a multicentre clinical trial conducted in the USA.21 In that study, children with CMPA, aged 2 months–4 years, were assessed by double-blind, placebo-controlled food challenges to HMO-supplemented w-eHF, or a currently marketed control w-eHF without HMO. Results showed that the percentage of subjects tolerating the test and control formulae was 98.4%, meeting clinical criteria for hypoallergenicity set out by the AAP.21,29
Nowak-Węgrzyn explained that this HMO-supplemented w-eHF was then further assessed in the CINNAMON study, a longer-term, double-blind, randomised, international, multicentre trial.30 In total, 194 infants were randomised 1:1 to receive either the test or control formula and, by 4 months, the per protocol groups included 73 test and 64 control subjects, respectively. The primary objective of the CINNAMON trial was to determine if weight gain was appropriate with the HMO-supplemented w-EHF compared with the control w-eHF without HMO, given the former’s reduced protein content.
Results from the CINNAMON study were positive, with similar average daily weight gain for the test and control groups confirming the non-inferiority of the HMO-supplemented w-eHF, despite lower protein content. Adequate growth was achieved in both groups, with no differences in key parameters such as weight for age z-scores or length for age z-scores between the HMO-supplemented formula versus control. Comparison of the Cow’s Milk-Related Symptom Score (CoMiSS™), which was used to track the symptom resolution across the time points of the study, also found no significant differences between the test and control group.30
Assessed as a secondary outcome in the CINNAMON study, Nowak-Węgrzyn described the impact of 2’-FL and LNnT supplemented w-eHF on infective morbidity as “very interesting.” From enrolment in the CINNAMON study to 12 months, a relative risk reduction was seen for various infections in infants fed the supplemented formula (Figure 1).30
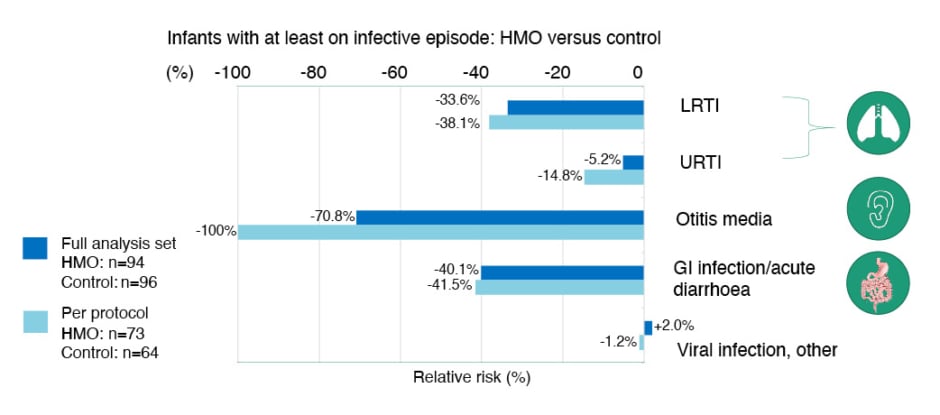
Figure 1: Relative risk reduction in infections (baseline to 12 months) in the CINNAMON study.30
Adapted from Vandenplas Y et al.30
EAACI: European Academy of Allergy and Clinical Immunology; GI: gastrointestinal; HMO: human milk oligosaccharide; LRTI: lower respiratory tract infection; URTI: upper respiratory tract infection.
Notably, there was a statistically significant reduction in the monthly frequency of upper respiratory tract infections (URTI) in the HMO-supplemented group versus control, together with a decreased incidence of ear infections at 12 months in the per protocol group.30 A non-significant, but clinically relevant, trend of 30–40% relative risk reduction in LRTI and gastrointestinal infections was also seen in those infants fed 2’-FL and LNnT-supplemented w-eHF.30
Nowak-Węgrzyn pointed out that these findings are in keeping with the reduced incidence of respiratory and gastrointestinal infections observed in healthy infants receiving HMO-supplemented standard infant formula. For example, one study revealed a significant 44% relative reduction in the risk of LRTIs in healthy infants fed formula with 2’-FL and LNnT from 2 weeks–12 months of age.5 Although the results for LRTIs in the CINNAMON study did not reach statistical significance due to sample size limitations, a 23% reduction in LRTI frequency per month was observed in infants with CMPA fed the supplemented formula. However, the study reported a highly statistically significant (p=0.003) 42% reduction in URTI episodes per month.30 The positive impact of HMO-supplemented w-eHF on URTI frequency is particularly important, stressed Nowak-Węgrzyn, because these are the infections driving unnecessary use of oral antibiotics in early life, and worsening gut dysbiosis.
Overall, the CINNAMON trial showed that 2’-FL and LNnT supplemented w-eHF formula supported normal growth, was effective at resolving allergy symptoms within 1 month, and led to infection reduction in infants with CMPA. This study suggests that Althéra® HMO has immune-enhancing properties, conferring a protective effect against respiratory and ear infections in infants with CMPA.30
Nowak-Węgrzyn went on to present results from the PLATYPUS study, which compared the growth of infants with moderate-to-severe CMPA fed an amino acid-based formula (AAF) containing 2’-FL and LNnT (Alfamino® HMO, Nestlé Health Sciences) to World Health Organization (WHO) growth standards.31 This single-arm Australian study involved 32 infants (average age: 18.6 weeks), who were followed to 12 months of age. At enrolment, infants in the PLAYTPUS study displayed clinical manifestations of either non-IgE or IgE-mediated CMPA, including eczema/atopic dermatitis (53.1%), urticaria (15.6%), and angioedema (6.3%). The most common symptoms at baseline were persistent crying/irritability (87.5%), frequent regurgitation/vomiting (62.5%), and persistent diarrhoea (59.4%).31
Infants in the PLATYPUS study who were fed the HMO-supplemented AAF achieved normal growth, with some evidence of catch-up growth. During the 4-month study period, all anthropometric parameters progressed along the WHO growth reference, with an upward trend from baseline to 4-month follow-up. There was also “prompt” CMPA symptom resolution, noted Nowak-Węgrzyn, with a significant reduction in the frequency of presenting symptoms from enrolment to the 1-month follow-up visit. The HMO-supplemented AAF demonstrated an excellent safety profile, and it was tolerated well by infants with moderate-to-severe CMPA. Only two participants experienced mild gastrointestinal adverse events that were deemed related to the study formula.31
In summary, HMOs play an essential role in supporting the developing immune system in infants who are breastfed, and these key nutrients should also be considered in infants who are not breastfed. Development of allergic disease is complex, and several factors contribute, including microbial dysbiosis, immune disturbances, and increased gut permeability, among others. The w-eHF with 2’-FL and LNnT was safe and well tolerated in infants with CMPA, plus it appears to reduce infective morbidity in the first year of life, possibly also reducing oral antibiotic use in these infants. Similarly, infants with moderate to severe CMPA who were fed an AAF with 2’-FL and LNnT achieved normal growth, with some catch-up growth, and the formula was well tolerated in this population. Clearly, these formulae are having a beneficial effect in children with CMPA, Nowak-Węgrzyn concluded.
Evolving Our Understanding of the Impact of 2′-Fucosyllactose and Lacto-N-neotetraose on the Gut Microbiome and Metabolome in Infants with Cow’s Milk Protein Allergy
Ralf Heine
Breast milk is the gold standard for infant nutrition and provides an unparalleled blueprint for formula development. The beneficial effects of breast milk on early immune and gut barrier function are largely driven by HMOs, Heine explained, which are important in the early establishment of an infant-type microbiome. Due to advances in biofermentation technology, it is now possible to produce hypoallergenic infant formula supplemented with the two breast milk identical HMOs, 2’-FL and LNnT, bringing formula composition closer to that of breast milk.32
The distinction between infant-type bifidobacteria and the large number of other bifidobacteria is an important one, stressed Heine. Infant-type bifidobacteria are comprised of four species (Bifidobacterum longum subspecies infantis, Bifidobacterium breve, Bifidobacterium bifidum, and Bifidobacterum longum subspecies longum), which are specialised in utilising HMOs, and are typical of the early colonisation of the infant microbiome. Lactate and acetate are the main metabolites of this HMO assimilation, and can be cross-fed to other bacteria which co-establish in the infant gut early on. These early coloniser species convert lactate to propionate and acetate to butyrate, the latter having strong tolerance-inducing and immune-modulating properties. Glycosyl hydrolases are bacterial enzymes that break down HMOs. The resulting breakdown products are shared between HMO-utilising bifidobacteria, and may “freeze out” other enteric bacteria via competitive nutrient depletion.33,34 Aromatic lactic acids produced by infant-type bifidobacteria are a more recent discovery. These compounds, which include indolelactic acid, act as important immune modulators for tolerance development, impacting on both T cell and monocyte responses.35
Having outlined the importance of infant-type bifidobacteria in establishing a healthy early-life microbiome, Heine went on to discuss results from the CINNAMON and PLATYPUS studies, assessing the impact of 2’-FL and LNnT on microbiome composition and metabolomic profiles in infants with CMPA (Boulangé et al., unpublished data).30,31 Stools were collected in the CINNAMON study at baseline, 1 month, 3 months, and 12 months. Shotgun metagenomics analysis was performed in 132 infants with CMPA aged 0.5–6.0 months, together with a faecal community type cluster analysis to assess temporal microbiome changes. A metabolome analysis, encompassing 137 metabolites, was performed in 84 infants.
Results showed preferential enrichment of infant-type bifidobacteria in the early enrolment cohort (aged 0.5–3.0 months at randomisation) of infants fed HMO-supplemented formula. Other bifidobacterial species, such as B. adolescentis, were not enhanced, suggesting that HMOs select out the “right bifidobacteria” for the infantile period, Heine explained. Looking at how the microbiome evolved over time, using a transition model of faecal community cluster types, revealed significant differences between test and control groups. In the late enrolment group (3–6 months of age at randomisation), approximately 50% of non-HMO supplemented infants had reached an adult pattern faecal community type 5 (FCT5) by 12 months compared with approximately 25% in the HMO supplemented group (Figure 2). Heine noted that remaining in an earlier, bifidobacteria-enriched microbiome pattern for longer may prolong the window period for early immune modulation, giving infants more time to develop normal immune responses.
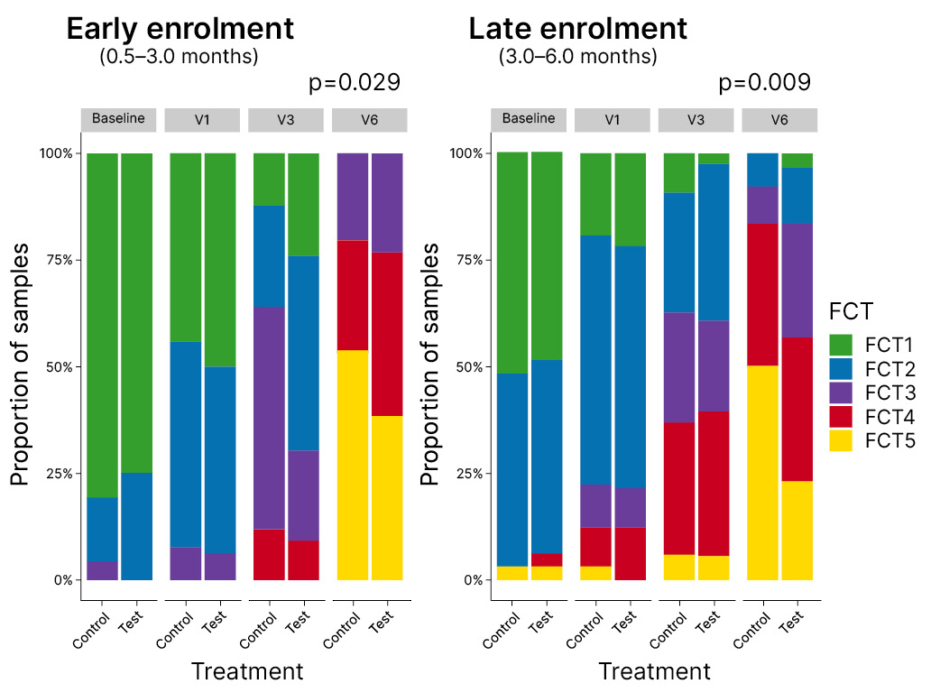
Figure 2: Transition model of faecal community cluster types in the CINNAMON study.
FCT5: faecal community type 5; V1: 1 month; V3: 3 months; V6: 12 months.
From baseline to 1 month, faecal acetate levels increased in the HMO-supplemented group and were reduced in control infants (p=0.09). No significant group differences in acetate signature were observed for subsequent visits but, as Heine explained, fermentation of dietary fibre may obscure the HMO effect once complementary feeding begins. Omics integration was also used to analyse alterations in 16 key metabolites and 17 KEGG orthologues, bacterial enzyme pathways, which were differentially expressed between HMO and non-HMO supplemented groups. Infants fed the HMO-supplemented formula displayed a decrease in conjugated bile acids, with upregulation of bile acid hydrolase activity up to 12 months of age compared with controls. A reduction was also seen in intermediates of branched-chain amino acid (Lys) and aromatic amino acid (Phe, Tyr, Trp) metabolism from 3 months to 12 months of age, indicating downregulation of oxidative protein catabolism via the Ehrlich pathway.
Faecal microbiome analysis was also carried out in the single-arm PLATYPUS study, where samples were available from 29 infants with CMPA at baseline, 1 month, 4 months, and 12 months.31 At baseline, infants showed a depletion of bifidobacteria. However, by 1 month, the microbial family of Bifidobacteriaceae were significantly enriched in response to HMO feeding, which was maintained to 12 months of age. At the phylum level, actinobacteria, including bifidobacterial, were enriched at 1 month, while abundances of potentially pathogenic proteobacteria were reduced.31
Looking specifically at HMO-utilising bacteria, significant enrichment was seen from enrolment in the PLATYPUS study to 12 months of age (Figure 3). Early amplification occurred from baseline to month 1 in the infant-type bifidobacteria, with a further strengthening of the bifidobacteria signal later on. A Taxon Set Enrichment Analysis was also performed on PLATYPUS samples, which revealed increased abundances of Bifidobacterium (1 month), Lachnoclostridium (Months 1 and 4), and Bacteroides (12 months) species, alongside a reduction in levels of Escherichia coli, Klebsiella, Streptococcus, and Rothia species.31 So, we can see a picture emerging of increases in early-stage HMO-assimilating bifidobacteria, and a reduction in proteobacteria, leading to partial correction of microbial dysbiosis, Heine explained. The SCFA profile showed that high levels of faecal acetate were maintained throughout the PLATYPUS study period, and butyrate levels significantly increased in the second half of the first year of life, potentially attributable to an HMO effect, with assimilation of acetate by butyrate-producing bacteria such as Faecalibacterium prausnitzii.31
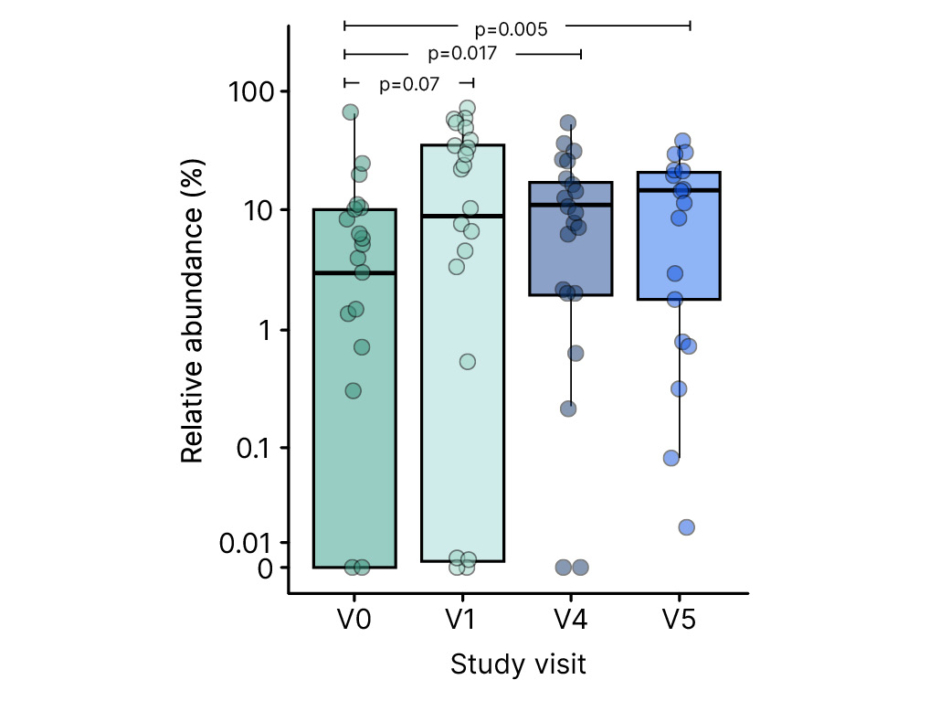
Figure 3: Enrichment of human milk oligosaccharides utilising bifidobacteria in the PLATYPUS study.31
V0: baseline; V1: 1 month; V4: 4 months; V5: 12 months.
In summary, these findings show that breast milk-identical HMOs promote an enrichment of the gut microbiome with infant-type bifidobacteria, and reduce the abundance of proteobacteria, suggesting a partial reversal of the microbial dysbiosis commonly seen in infants with CMPA. HMO supplementation slowed the premature shift to an adult-type microbiome often seen in infants who are not breastfed, which may prolong the window period for early immune modulation. The metabolomic analysis showed an early increase in faecal acetate, as well as HMO-related changes in bile acid deconjugation and oxidative amino acid catabolism. The full clinical significance remains to be elucidated, and further well-designed studies on the immune-modulating effects of breast milk-identical HMO in infants with CMPA are needed, Heine concluded.
Question and Answer Session
A short question and answer session followed the main symposium, in which panel members were posed questions by the Chair and members of the live audience.
Santos asked at what stage of immune development HMOs are most important, particularly in infants who are not breastfed. Nowak-Węgrzyn replied that formula supplemented with HMOs should be introduced during the first 6 months of life, adding “the earlier, the better.”
A member of the audience questioned the rationale for mimicking breast milk with HMO-supplemented formula, given that breastfeeding itself is not protective against food allergy. Heine acknowledged that “it’s a complex picture,” but stressed that the immunomodulatory effects of breast milk and HMOs on the early life microbiome constitute “the best approach that we currently have” to protect against allergic disease and to avoid prolonged dysbiosis. For CMPA in particular, the aim is for outgrowth, so diagnosis and intervention have to come early, Heine added.
A further audience question asked about the influence of maternal diet on the abundance of protective HMOs in breast milk. Diet plays a role, agreed Heine, as do genetic factors, with approximately 20% of mothers known to be non-secretors of 2’-FL. Different stages of breast milk may also confer different HMO compositions, so trying to mimic all this in formula is a “big ask,” Heine acknowledged. However, very encouraging results have been seen in the infection space already, Heine noted, and the “next frontier” will be tolerance development.
Asked about innate and acquired immune mediators in collected stool samples, Heine pointed to aromatic lactic acids, which have emerged as new immune modulatory candidates, and noted that, together with butyrate and other SCFAs, these could act as useful metabolic markers of tolerance development.
Finally, Santos asked about the immune mechanism underlying HMO effects on the reduction of infection and medication use. O’Mahony explained that the epigenetic landscape of immune cells is critical early in life. Factors that promote the “opening up” of the infection countering Th1 immune response and the “closing” of Th2 response to protect against allergy is critical, O’Mahony added, with many factors playing a role, including bifidobacteria and SCFAs.





