Meeting Summary
The aim of the symposium was to share learnings from the recently established European Academy of Allergy and Clinical Immunology (EAACI) Task Force on special products for cow’s milk protein allergy (CMPA), with the intention of providing an overview on controversies regarding extensively hydrolysed formulas (eHFs), their utility, and the validity of the definition ‘special products for CMPA’.
Dr Rosan Meyer opened the symposium by discussing the evidence for appropriate dietary management in CMPA, emphasising the importance of breastfeeding and dietary management of breastfed children with CMPA, hypoallergenic formula, and the current controversies and debate around formula choice. Dr Martinas Kuslys covered the current interpretations and ranges for definitions for eHFs, and presented data from an analytical programme that aims to improve understanding of the wide range of commercially available formulas, with the objective of defining eHFs in a more consistent, meaningful, and practical way. Prof Antonella Muraro and Prof Arne Høst closed the session with a discussion around the need for updated guidelines to ensure safe products for infants with CMPA, summarising some of the issues with currently available hypoallergenic formulas.
Welcome and Introduction
Professor Antonella Muraro and Professor Arne Høst
The EAACI Task Force on products for CMPA was established in 2016, with the objective of addressing the need for improved knowledge regarding treatment of infants with CMPA. The symposium speakers are members of the EAACI Task Force, which aims to better define eHFs through a collaboration between academia and industry. The ultimate goal is to provide clarity in the field and to offer safe and suitable solutions, and advice for daily clinical practice.
Choosing the Most Appropriate Dietary Management for Infants with Cow’s Milk Protein Allergy
Doctor Rosan Meyer
There are currently two existing definitions for ‘hypoallergenic formulas’.1,2 The first definition for specialty infant formulas with reduced allergenicity is based arbitrarily on a content of <1% immune-reactive protein of total nitrogen, while the second is based on the product being tolerated by at least 90% of infants (with 95% confidence interval) with documented CMPA. A key feature of the second definition is the recommendation that after a successful double-blind challenge, clinical testing should also include an open challenge, using an objective scoring system to document allergic symptoms during a 7-day period. A controversial aspect of the first definition is the lack of supporting evidence that the <1% threshold would prevent a clinical reaction. Therefore, there has been a drive by official bodies for hypoallergenic formulas to be tested in clinical trials and to comply with the second definition.
There is a lack of consistency around the definition of the Dalton size of peptides in eHF. A proposal used in many guidelines1,3 dictates that the product should have free amino acids and peptides <1.5 kDa in size. This proposal may have originated from a study of peptide lengths in commercially available formulas, in which significant amounts of peptides of molecular weights (MWs) >1.5 kDa were not detected in any of the tested feeds.4 The study authors did not recommend using >1.5 kDa as a cut-off, they simply reported that the formulas they tested did not contain significant amounts of larger proteins; however, various subsequent publications have featured it as a recommendation. Interestingly, a review of clinical studies of different formulas found that Dalton size alone does not predict clinical outcome.5
eHFs are suitable for most infants with CMPA,6 and can contain protein derived from casein, whey, or rice. Casein-based eHFs were one of the first established hypoallergenic formulas (>60 years ago), while whey-based eHFs have been available since the early 1990s, and can contain lactose. Many casein and whey-based eHFs are well-established and tested products. However, many products currently available on the market have not been subjected to rigorous testing. Extensively hydrolysed or partially hydrolysed rice formulas are relatively new, and are not available worldwide. Rice-based eHFs have undergone testing according to the EAACI guidelines in two studies.7,8 However, limited data exist on the effect of rice-based formulas on growth and its nutritional adequacy, with only a small number of studies available, featuring low numbers of patients. A question also remains over the presence of arsenic in rice-based formulas.9
Amino acid-based formulas (AAF) contain proteins only in the form of individual amino acids, and none are based on any cow’s milk proteins. Generally, AAFs are reserved for the subgroup of patients with the most severe cases of CMPA6 as they are considered the only truly non-allergenic formulas, with products available for infants both <1 year and >1 year of age. A recently submitted systematic review concluded that the following conditions warranted the use of AAF: failure on an eHF, eosinophilic esophagitis, faltering growth and multiple food eliminations, and anaphylaxis.10
Lactose has numerous benefits as an ingredient in formula. Historical fears regarding the risk of adverse reactions to lactose, as expressed in a 1999 joint statement of the European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) and European Society for Paediatric Allergology and Clinical Immunology (ESPACI),2 have been reassessed. More recently, ESPGHAN stated that: “adverse reactions to lactose in CMPA are not supported in the literature, and complete avoidance of lactose in CMPA is no longer warranted. eHFs containing purified lactose are now available and have been found safe and effective in the treatment of CMPA”.11
It is important to note that lactose is the main source of carbohydrate in breast milk.12 Given that in non-immunoglobulin (Ig)E-mediated gastrointestinal allergies, breastfeeding is strongly recommended,13 concerns over lactose seem unfounded. In addition to the positive impact of lactose on taste, lactose has a beneficial effect on the gut microbiota and metabolome in children with CMPA,14 and has also been shown to improve the absorption of calcium and zinc.15
The need for a multidisciplinary approach in managing CMPA is summarised by a statement from the Italian Society of Paediatric Nutrition/ ItalianSociety of Pediatric Allergy (SINUPE/SIAIP) Allergy and Immunology Task Force:16 “the interaction of the nutritionist, dietitian, nurses, allergologist and, whenever possible, psychologist, is the most successful way to ensure both growth and health of allergic children”. Dietitians, as part of a multidisciplinary team, are known to improve parents’ experience, reduce the number of appointments, and increase cost efficiency.17 Dietary counselling has also been proven to result in a significant improvement in anthropometric and laboratory biomarkers of nutritional status of allergic children.18
In summary, there are several factors which need to be taken into consideration when choosing the most appropriate dietary management for infants with CMPA. Many guidelines exist for the management of CMPA, which recommend eHF for most cases as first-line formula, and AAF for the severe spectrum of CMPA.6 When making the choice, it is also important to consider not only the nutritional status of the child, but also whether the hypoallergenic formula has been tested in children with CMPA as required by EAACI guidelines, if growth and nutritional adequacy data have been published, and if the micronutrient content is suitable for the child. For example, medium-chain triglycerides can optimise absorption of lipids in patients with malabsorptive disorders, and the addition of iron in follow-on formulas and formulas suitable for >1 year can be considered. The addition of vitamins, prebiotics, and probiotics, as well as taste and flavour additions, are also important to consider.19,20 Practicalities, such as local availability, the reimbursement environment, and cost of formula must also be taken into account. The age of the child can also impact formula choice and taste acceptance, and religious and other dietary considerations (e.g. presence of multiple food allergies or being vegan/vegetarian) may restrict the formula choice further.
Not All Extensively Hydrolysed Formulas Intended for Cow’s Milk Protein Allergy are the Same
Doctor Martinas Kuslys
Although the ultimate goals of all eHFs are the same, to be well-tolerated by most infants with CMPA and to be nutritionally complete with similar taste and consumption properties to regular formulas, recent publications have highlighted the chemical heterogeneity of eHFs.21,22 Currently, eHFs can be characterised by either chemical analysis3,23 or by the desired clinical outcome.11,24,25
Chauveau et al.22 analysed the peptide profiles of three whey-based eHFs. Each peptide profile was found to be different. Two were found to have residual whey peptides, recognised by specific IgE, and two had residual caseins.22 The authors concluded that: “the degree of hydrolysis and the size of residual peptides of each eHF should be known by practitioners”.22 In another study, four peptide profiles were tested from four batches of several commercially available casein-based eHFs and each was shown to be different. The authors concluded that dissimilarities in peptide profiles of the products may be related to the differences in their overall functionality.21 These functional differences have also been observed in clinical practice. Although these observations cannot be generalised for all eHFs, in a study of 49 children with CMPA, half were found to have incomplete resolution of symptoms following whey-based eHF treatment.26 Surprisingly, few eHF products have been shown to be efficient in terms of both allergy and growth.
Samples of commercially available eHFs from 12 countries (sourced from 11 major suppliers) were analysed with a clear focus on suitability for the management of CMPA. The programme consisted of internal investigations at the Nestlé Research Labs, Switzerland and external investigations at Neotron SPA, Italy and was conducted in accordance with accepted international testing standards. Only eHFs based on cow’s milk proteins and marketed for the management of CMPA in infants were included. Although the MW distribution of hydrolysates can be used as an indicator of the degree of hydrolysis, several other parameters should be used to characterise eHFs. Quantification of residual proteins is also important as values reflect both the design of the formula and the quality management in production. The analysis comprised of osmolarity, nitrogen fractions, lactose content, total and free amino acids, β-lactoglobulin, and casein content and included sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and size exclusion-high-performance liquid chromatography (SE-HPLC) for peptide profiling and MW distribution analysis. The results focussed on three main aspects: peptide profiling and MW distribution, and both β-lactoglobulin and casein content by enzyme-linked immunosorbent assay (ELISA).
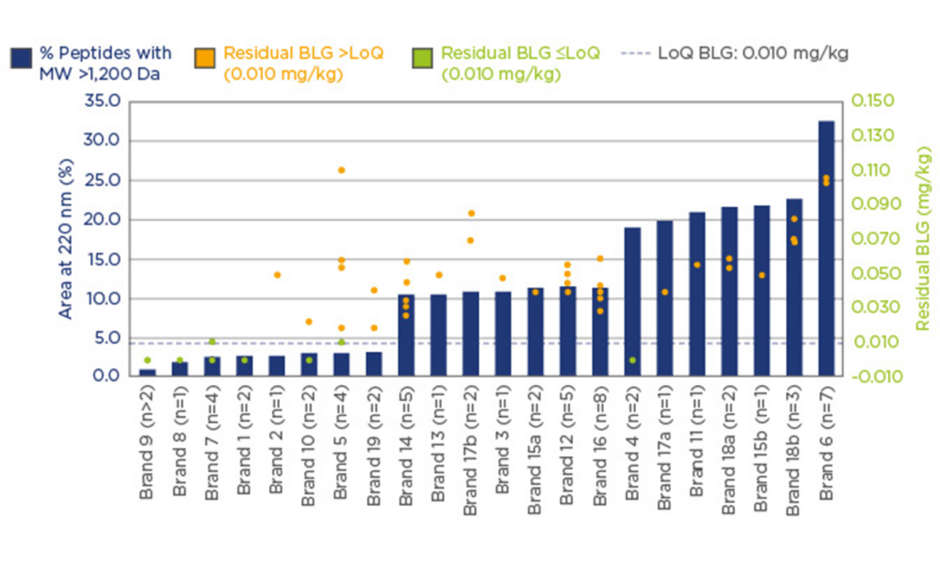
Significant variation in the peptide MW distribution was found, and the percentage of peptides with MW >1.2 kDa varied from 1–36%. Similarly, β-lactoglobulin levels were found to vary by more than two-orders of magnitude. eHF samples were grouped into two categories; 20% of investigated samples had non-measurable β-lactoglobulin content (lower than or at the limit of quantification [LoQ]: 0.010 mg/kg), and 80% of samples had β-lactoglobulin content >LoQ, with high variability from 0.020–36 mg/kg.
Casein concentrations also displayed a similarly wide variation. With 83.3% of samples having non-measurable casein content (≤LoQ: 0.2 mg/kg), and 16.7% having casein content >LoQ, with high variability from 0.3–1.1 mg/kg. Figure 1 displays these data and highlights the importance of using a combination of analytical methods when carrying out assessments of formulas. The results suggest that a high degree of hydrolysis is desirable, but further quality control elements are needed to ensure consistently clinically safe products.
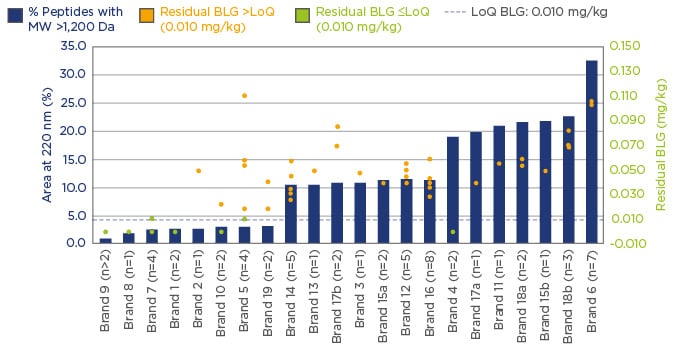
Figure 1: A combination of analytical methods; assessing formula suitability for the management of CMPA.
BLG: β-lactoglobulin; Da: Dalton; LoQ: limit of quantification; MW: molecular weight; CMPA: cow’s milk
protein allergy.
It has been recognised by EAACI that not all eHFs are clinically tested or fit for their intended purpose.6 The wide variation of the degree of hydrolysis in commercially available eHFs reflects the lack of alignment for the definition of ‘extensively hydrolysed’. The high variability of commercially available eHFs and wide interpretation of the definition of eHF results in some products which are perhaps incorrectly labelled as ‘extensively hydrolysed’ and may be unsuitable for CMPA management. In 2010, the Spanish Food Standards Agency (AESAN) disclosed that milk protein had been found in samples of baby milk that had been marketed as being suitable for infants with milk allergies, leading to a product recall.27 These findings highlight that the degree of hydrolysis alone, is not sensitive enough to characterise eHFs. It is recommended that actionable guidelines should be introduced and implemented to better define eHFs, and to provide guidance on conducting clinical trials.
New Guidelines Ensuring Safe Products for Infants with Cow’s Milk Task Force on Special Products for Protein Allergy: Update from the EAACI Cow’s Milk Protein Allergy
Professor Antonella Muraro and Professor Arne Høst
At present, not all commercially available products for infants with CMPA are safe and effective, since some contain a substantial proportion of high MW peptides, with a variable degree of residual antigenicity and allergenicity.28-30 The criteria for hypoallergenic formulas recommended by EAACI in 2004 states that hypoallergenic formulas should have 90% clinical tolerance (with 95% confidence interval) in infants with IgE-mediated CMPA. Furthermore, the formula should be investigated in at least two centres with consecutive patients representing both IgE and non-IgE-mediated CMPA.31 Casein hydrolysates,32,33 whey hydrolysates,34-37 and amino acid mixtures35,38-41 have been shown to meet these criteria; however, there have been reports of allergic reactions to formulas labelled as ‘eHF’,22 suggesting they do not meet these criteria and are neither safe nor effective.
The degree of hydrolysis and content of β-lactoglobulin has been investigated in a range of products.30,42,43 Currently, there is no unanimous agreement on the criteria for eHF classification. Products can only be defined as non-allergenic if they are pure amino acid mixtures, all others, even those labelled as hypoallergenic, contain residual allergenicity and may induce allergic reactions.
Reduction of allergenicity can be achieved through several processes which can be combined, such as, enzymatic hydrolysis, heat treatment, and ultrafiltration. Hypoallergenic formulas can contain residual antigenicity due to inadequate hydrolysis and filtration, the presence of peptides with cow’s milk protein (CMP)-derived epitopes, the aggregation of smaller peptides, and the cross-reaction of epitopes with those of CMP. Contamination and inclusion of other antigens can be introduced during production or packing processes, and from carbohydrate and lipid sources, which may explain batch-to-batch variations. Many products defined as ‘hypoallergenic’ have also undergone changes in their chemical composition, such as the addition of probiotics and prebiotics, long-chain polyunsaturated fatty acids, and other additives. It is unclear how these changes impact the ‘hypo-allergenicity’ as evaluated in the initial product.
Numerous tests are available to determine the antigenicity of cow’s-milk-based formulas, including: physicochemical tests, which allow formal titration and an estimate of the percentage of hydrolysis, SDS-PAGE for MW determination, immunoblotting, inhibition ELISA and radioimmunoassay (using sera from sensitised patients), and animal models of anaphylaxis.
Future developments in the field should include industry-wide agreements on standards for preclinical testing, and quality control and assurance. Careful clinical testing, should also be carried out for quality assurance of each new ‘hypoallergenic’ product before its launch in the market. Strict criteria should be established with requirements for informative labelling on all products, and a European database of products for CMPA should be created, allowing information on adverse reactions to be collected.