INTRODUCTION
Hereditary angioedema (HAE) is a rare genetic condition characterised by unpredictable episodes of cutaneous or submucosal swelling in different parts of the body, which can be life-threatening when affecting the upper respiratory tract.1
People with HAE are often highly anxious due to the burden of illness and treatment they face, and this is compounded by the complex decision-making process they face every time they experience the first signs of an attack.
Treatment guidelines recommend that all patients with HAE always carry on-demand treatment, to use if they experience an attack.1 The guidelines also state that all HAE attacks should be treated as soon as possible.1
However, current on-demand treatments are administered parenterally, either intravenously or subcutaneously, and are associated with limitations that make it difficult for patients to follow treatment guidelines. As a result of delayed or non-treatment, patients continue to experience ongoing attack burden that negatively impacts their quality of life.
In this sponsored symposium, Chair Douglas Jones, Rocky Mountain Allergy, Asthma, and Immunology, Layton, Utah, USA, was joined by fellow HAE experts Thomas Buttgereit, Charité – Universitätsmedizin Berlin, Germany; Emily Carne, University Hospital of Wales, Cardiff, UK; and Danny Cohn, Amsterdam University Medical Center, the Netherlands.
Through a series of short presentations, discussions, and audience Q&A, the panel highlighted how better communication between healthcare professionals (HCP) and patients could encourage more patients to carry on-demand treatment all of the time, and treat all attacks early. Panel members also discussed how orally administered on-demand treatments, currently being tested in clinical trials, could help patients to treat attacks more quickly, and easily, and improve quality of life.
CHALLENGES WITH DELAYS IN ON-DEMAND TREATMENT
Douglas Jones
Jones opened the symposium by articulating the decision-making process that someone with HAE would go through if they entered the conference hall and started to experience a slight tingle in their lip or cheek. He described how this would be enough to start them worrying whether they were about to experience an HAE attack. They might be in conversation with a colleague, but at the same time, they would have to decide whether they should excuse themselves to find a private space to administer on-demand treatment, and potentially miss the opening presentation.
This typifies the dilemma everyone with HAE faces day-to-day, and contributes to feelings of anxiety and frustration, Jones explained.
Prompt treatment of HAE attacks is associated with a shorter time to resolution of symptoms and shorter total attack duration, regardless of attack severity.1,2 The current international World Allergy Organization (WAO) and European Academy of Allergy & Clinical Immunology (EAACI) treatment guidelines recommend that attacks are treated as early as possible, and that all patients have sufficient on-demand medication to treat at least two attacks and always carry on-demand medication.1
However, Jones asked: “Are patients following these guidelines?” Studies show that only 36% of patients always carry their on-demand treatment, and travel an average of 3.5 hours from home without their medication.3 Patients can delay up to 3.8 hours before treating attacks with on-demand medicine (KalVista Pharmaceuticals, unpublished data) and more than 40% of attacks remain untreated.4
“Clearly, there is a massive gap between what the guidelines say and what patients actually do,” Jones emphasised, inviting the panel to discuss this further, via a series of specific questions.
Why do patients delay, or forgo, treatment for HAE attacks?
People with HAE prefer to self-administer treatment in a private space. They would rather do so at home, and many do not carry the medication with them when they go out, Buttgereit explained. He added: “Individuals may have issues with the parenteral route of administration of the treatment,” and some experience injection site reactions. Others need help with injections and may have to attend hospital. This can lead to further treatment delays and contributes to the burden of treatment.
In addition, Carne said myths circulate among patients about when to treat attacks. “In the past, patients have been told to ‘wait and see’ and hold off with treatment. As clinicians, we need to dispel those myths.”
Cohn concurred, adding that previous guidelines recommended treating only severe attacks. “It is important that at every clinic visit, we remind patients that, in line with current guidelines, all HAE attacks should be treated as early as possible.”
Carne added that it is important to understand from each patient what their own personal reasons for delaying treatment are. They gave the example of a patient who has a needle phobia and will avoid both hospitals and self-treatment at all costs.
Jones agreed, saying: “In order for patients to trust us, and to trust the recommendations we are giving, we have to listen, so we know where they are and where we can guide them to.”
How do you discuss the benefits of treating attacks early with your patients?
“As nurses, we often have more time with our patients than the physician. We can listen to them and understand their life and the difficulties they face,” Carne said. This knowledge helps to create a shared care environment with collaborative decision-making between the healthcare team and the patient.
“It is also very important to help the patient understand that it is not risky to treat early. It is riskier to not treat when you should have done,” Carne emphasised.
Cohn agreed, adding: “Often patients will say, ‘This discomfort in my abdomen could be something else, let’s just wait.” When they finally decide to treat the attack, it has evolved, and the symptoms may be more severe and take longer to resolve. Patients need to be reassured that there are no significant risks or side effects to taking currently licenced drugs.
The panel agreed that patient education is an ongoing process and conversations around when and how to treat attacks should take place at every clinic visit. Buttgereit highlighted the value of HAE specialist centres: “Here, the whole team, from the nurse to the physician, give the same information.”
What are the key takeaway messages and the call to action for HCPs who care for patients with HAE?
“The sooner the better for treatment,” Cohn reiterated. HAE is a potentially fatal condition. Early treatment allows patients to lead a normal life and do activities as and when they want to.
Buttgereit agreed and added that all HCPs should understand that HAE is a severe disease that affects quality of life and causes significant anxiety to patients. “All physicians should follow the guidelines: ‘Treat early, treat every attack,”1 Buttgereit said.
Jones went on to reiterate that HAE provokes anxiety in patients, because of the burden of disease and the burden of treatment. He described the patients’ attack journey (Figure 1), from trigger to attack progression and treatment.
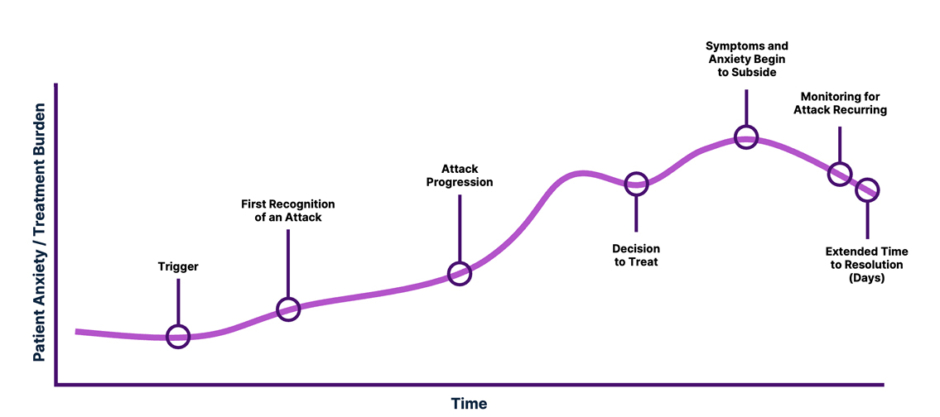
Figure 1: Illustrative impression showing anxiety and on-demand treatment burden during a hereditary angioedema attack.
The unpredictability of attacks and anticipation of on-demand treatment creates anxiety. The life-threatening nature of this condition also creates anxiety. Once triggered, patients often wait before treating an attack, asking themselves questions about the implications of treating or not treating. If the patient self-treats, they need to remain vigilant to check that symptoms subside, and at the same time, watch out for recurrences. Then, the attack itself may take multiple days to resolve (Figure 1).
Treating attacks early can help patients regain some control, explained Jones. “The decision to treat can make the situation more predictable. From that moment, the patient can take control, which helps to reduce anxiety.”
HAE ON-DEMAND TREATMENT CLINICAL TRIAL LANDSCAPE
Danny Cohn
The landscape for on-demand treatment for HAE is changing, explained Cohn. “We are facing a paradigm shift in on-demand treatments for HAE that are easier to administer,” they said. The first C1-inhibitor concentrates to treat HAE attacks were developed more than 40 years ago and until now, all on-demand medications have been administered parenterally. “Now we are seeing new products in clinical trials, including oral drugs to treat angioedema attacks,” Cohn explained. They then outlined recent and ongoing clinical trials of two new oral on-demand medications, deucrictibant and sebetralstat.
Deucrictibant
Deucrictibant targets the bradykinin B2 receptor to inhibit swelling in HAE.5 In a randomised, double-blind, placebo-controlled cross-over Phase II clinical trial, RAPIDe-1 study, 74 patients from 13 countries were enrolled and treated with study drug or placebo.6 Primary and key secondary endpoints were met.6 HAE symptoms were reduced, and it took less time to relieve symptoms and resolve attacks, compared to placebo.6 There was also a reduction in the use of rescue medication and the drug was well tolerated.6
Sebetralstat
Sebetralstat is an oral plasma kallikrein inhibitor with rapid absorption after ingestion.7 The results of a Phase III randomised, double-blind, placebo-controlled trial of sebetralstat (KONFIDENT) have now been published.7 This was a 3-way cross-over study, with 136 patients from 17 countries randomised. Patients self-treated up to three HAE attacks with either 300 mg sebetralstat, 600 mg sebetralstat, or placebo.7 The trial was event-driven and there were 264 attacks before the trial was finalised.
Primary and secondary end-points were measured using patient-reported outcomes on the seven-point Patient Global Impression of Change (PGI-C) scale, and the five-point Patient Global Impression of Severity (PGI-S) scale. The primary endpoint was time to the onset of symptom relief, defined as a rating of ‘a little better’ on the PGI-C scale at two or more consecutive time points within 12 hours after the first administration of the trial agent.7 Secondary endpoints included time to reduction in attack severity and time to complete attack resolution.7 These were defined, respectively, as an improved rating on the PGI-S scale, with ratings ranging from ‘none’ to ‘very severe’, at two or more consecutive time points within 12 hours, and complete attack resolution (a rating of ‘none’ on the PGI-S scale) within 24 hours.7
More than half of patients self-administered the study medication within 1 hour of an attack starting, and a quarter of patients self-administered within 6 minutes.7 The time to beginning of symptom relief was much shorter in both sebetralstat groups (1.6 hours for 300 mg dose and 1.8 hours for 600 mg dose) compared to patients taking the placebo (>6 hours).7 In addition, the observed safety profile of sebetralstat was equivalent to that of placebo.7 In an ongoing open-label extension trial, KONFIDENT-S,8 initial data suggests that the median time from attack recognition to treatment is 9 minutes.8 Cohn finished his presentation by asking colleagues on the panel the following question:
Do you think the barriers to treatment for an oral drug are going to be different for patients when thinking about very early symptoms?
Jones responded by reminding the audience of the scenario of someone with HAE attending this symposium who is faced with a choice between leaving the discussion to find a private space to inject/infuse medication, or being able to take a pill. “I think having an orally administered treatment changes the dynamic of when and how early to treat, and removes a barrier for patients,” they said.
Carne reiterated that patients already carry a burden of illness; therefore, anything that reduces the burden of treatment is important. “Removing a needle is reducing the burden of treatment,” Carne said.
“Being able to take a tablet means you don’t have to identify yourself as being someone with HAE,” Buttgereit added. Cohn also commented, saying: “It also gives patients more independence. They do not need to rely on HCPs or family members to help them.”
AUDIENCE Q&A
Jones invited questions from the audience, which included the following:
Currently, if we treat prodromal symptoms of an attack, it is off-label treatment. Should we redefine what an HAE attack is?
Carne acknowledged this is an important question and said, “If we are not clear on when to treat, how can we advise our patients?” Cohn’s view is that patients know when an attack is starting, and early signs might be invisible, for example, manifesting as fatigue, or irritability. “We need to define what a prodrome is. Why wait for a full-blown attack when you know the prodrome is going to evolve into an attack?”
It can be a challenge to persuade less experienced colleagues to advise patients to treat early. Do you think an oral medication will help remove a psychological barrier to treat?
“Yes,” Carne said. “The threshold for treating with a tablet is going to be lower than for an injection.”
Buttgereit added: “We are trained to believe that intravenous treatment is the fastest way to treat, but now, on the horizon, we have something that is just as quick.” Cohn reminded the audience that it is the type of drug that ensures rapid effect, not the route of administration.
How will guidelines evolve as the paradigm shifts to early treatment of all attacks?
“Treat at the very first sign of anything that may lead to an attack,” Cohn suggested. The threshold to treat should be as low as possible. Carne added that “patient power” is also key. “We have excellent HAE patient networks around the world and when we see treatments working, the pressure will be on to update the guidelines to reflect that.”
Will these new treatments change your recommendations to patients on taking long-term prophylactics (LTP)?
“That is difficult to predict, but at the moment, no, I don’t think so,” Buttgereit asserted. “The current guidelines recommend that treatment of HAE should be individualised, and all patients should be considered for LTP and carry on-demand treatments. Some patients experience breakthrough attacks despite being on LTP, so it is necessary to always keep on-demand treatment with you,” Buttgereit added. In addition, said Cohn: “Those patients that are otherwise well-controlled with infrequent, mostly mild attacks, may discontinue LTP if an oral on-demand option is available.”
CONCLUSION: HOW DO WE CLOSE THE COMMUNICATION GAP IN HEREDITARY ANGIOEDEMA MANAGEMENT AND HELP OUR PATIENTS ADDRESS DELAYS IN ON-DEMAND TREATMENT?
The panel’s consensus view was articulated by Jones when he said: “We, as HCPs, need to communicate with patients, educate them, hear them, acknowledge them, help them feel understood. Empower them.”
Jones then summarised the discussions (Figure 2). Patients currently delay and forgo on-demand treatment, and yet we know they can benefit from treating attacks early. Faster time to attack resolution and recovery is important to patients,9 and new oral on-demand medicines are in the pipeline that should help to address this.6,7 All HCPs should ask their patients about the barriers they face to treating all attacks as early as possible; then, reinforce them to treat early, and emphasise the need to carry on-demand treatment at all times.

Figure 2: Summary.





