Chairpeople: Angela Claver Monzón1
Speakers: Andrea Mikkelsen,2 Kari Nadeau,3, Wendy Sue Swanson3,4
1. Hospital Universitari Quiron Dexeus, Barcelona, Spain
2. University of Gothenburg, Sweden
3. The Sean N. Parker Center for Allergy and Asthma Research, Stanford University School of Medicine, California, USA
4. Before Brands, Inc., Menlo Park, California, USA
Disclosure: Monzón reports speaker fees and/or honoraria from Nestlé Health Science, Nutricia, Leti Pharma, and Diater. Mikkelsen has received honoraria from Nestlé Health Science. Nadeau has received grants from National Institute of Allergy and Infectious Diseases (NIAID), Food Allergy Research & Education, End Allergies Together, Allergenis, and Ukko; personal fees from Regeneron, AstraZeneca, Immuneworks, and Cour; is a member of the Data and Safety Monitoring Board at Novartis and National Heart, Lung, and Blood Institute (NHBLI); is a Co-founder of Before Brands, Alladept Immunotherapeutics, IgGenix, and ForTra; and has been a consultant/advisory board member for Ukko, Before Brands, Alladapt, IgGenix, Probio, Vedanta, Centecor, Seed, Novartis, NHBLI, and the Environmental Protection Agengy (EPA); and has been on the Network Steering Committee the of Immune Tolerance Network and Board of Scientific Counsellers of the National Institutes of Health (NIH) Programs. Swanson is an employee and shareholder of Before Brands, Inc.
Acknowledgements: Medical writing assistance was provided by Steph Carter, Lyrical Medical Writing Services, Manchester, UK.
Support: The symposium and the publication of this article were funded by Nestlé Health Science. The views and opinions expressed are those of the authors and not necessarily of Nestlé Health Science.
Citation: EMJ Allergy Immunol. 2022;7[1]:33-41. DOI/10.33590/emjallergyimmunol/10046575. https://doi.org/10.33590/emjallergyimmunol/10046575.
Meeting Summary
This symposium occurred during the European Academy of Allergy and Clinical Immunology (EAACI) Congress, 2022. Angela Claver Monzón from the Hospital Universitari Quiron Dexeus, Barcelona, Spain, welcomed attendees and gave a brief introduction to the topic of food allergy prevention in children. Andrea Mikkelsen from the University of Gothenburg, Sweden, highlighted the importance of diet diversity and the early introduction of food allergens to infants before discussing some of the challenges associated with this. Kari Nadeau from The Sean N. Parker Center for Allergy and Asthma Research, Stanford University School of Medicine, California, USA, described the results from a study that evaluated different strategies for the early introduction of food allergens. She highlighted that consumption of even small quantities of a multi-allergen mixture for 1 year was associated with improvements in subsequent food challenge reactivity compared with single or double food introductions. Wendy Sue Swanson, also from The Sean N. Parker Center for Allergy and Asthma Research and Before Brands, Inc., outlined the development of a 16-allergen mixture and described the rationale and design of the INTENT study, which will evaluate the potential benefits of this product to support early food allergen introduction. The symposium concluded with a live question and answer session.Welcome and Introduction
Angela Claver Monzón
Food allergies are a growing health epidemic, with population-based surveys in the USA estimating that up to 8% of children and 11% of adults are now living with a food allergy.1,2 During the 1990s and early 2000s, international guidelines recommended the avoidance of commonly problematic food during infancy due to the belief that early introduction of these foods may increase the risk of allergies. However, beginning with the publication of the LEAP trial in 2015,3 a paradigm shift in the understanding of food allergy prevention has occurred. Clinical guidelines now generally recommend the introduction of potentially allergenic food after 4–6 months of exclusive breastfeeding.4-6
Real-World Experience in Early Food Allergen Introduction
Andrea Mikkelsen
Food allergies are one of the most common chronic diseases in early childhood,7-9 requiring clinicians and dieticians to commit considerable time to their prevention, diagnosis, and management. For babies, the transition from breast or bottle feeding to eating solid food can be challenging and requires learning a completely different feeding technique. Mikkelsen highlighted that infants typically triple their birth weight during the first year of life and then further double their weight by the age of 6 years. During this time, children must develop good eating skills and consume a diverse and nutritious diet to support growth and prevent disease. For this reason, there are guidelines to support infant feeding, which all now recommend early rather than the delayed introduction of food allergens to prevent food allergies.4-6
The importance of the early introduction of food allergens is supported by data from a recently published study, which randomised infants (n=2,397) from a general population into a food intervention group (early complementary feeding of peanut, cow’s milk, wheat, and egg from 3 months of age); a skin intervention group (skin emollients from age 2 weeks–<9 months); a combined intervention group (food and skin interventions); or to a no intervention group.10 In this study, food allergies at 36 months of age were diagnosed in 0.9% of infants in the food intervention group and 1.2% of the combined intervention group, compared with 3.0% of the skin intervention group and 2.3% of the non-intervention group.10 Overall, there was a significant reduction in the prevalence of food allergy in the food intervention group compared with the no food intervention group (risk difference: -1.6% [95% confidence interval: -2.7–-0.5]; odds ratio: 0.4 [95% confidence interval: 0.2–0.8]).10 Mikkelsen emphasised the importance of this study of infants from a general population, as food allergies often develop in children without known genetic risk factors for food allergies. Mikkelsen also highlighted the importance of diet diversity to prevent food allergies,4,11-13 including a recently published study by Venter et al.,14 which demonstrated that consuming vegetables and yoghurt during pregnancy could reduce the risk of any allergy in offspring.
Mikkelsen also raised the issue of reduced diet diversity and dysbiosis, which is a microbial imbalance in the gut.15 In addition to food allergies, dysbiosis can result in other inflammatory conditions, including skin conditions, inflammatory bowel diseases, functional gastrointestinal disorders, and neuropsychiatric disorders.16 The gut microbiota may play a protective role throughout life and not just in infancy and childhood. This is illustrated by a study in twins, which demonstrated significant differences in fecal microbiomes and metabolomes in twin pairs throughout adulthood.17
Mikkelsen acknowledged that the early introduction of food allergens can be challenging for infants, caregivers, and clinicians.18-22 It is important to note that parents and caregivers were typically born and raised when food avoidance was recommended. As a result, the knowledge and experience of infant caregivers are now being challenged, and their acceptance of the new guidance may require significant education and support. The impact of these challenges is illustrated by a recent survey in the UK, which showed that many caregivers continue to delay the introduction of food allergens to their infants beyond 6 months of age (Nestlé Health Science, unpublished data). It was noted that dairy and oats are typical weaning foods and are hard to avoid; however, the introduction of other food allergens typically requires an active decision to be made.
Mikkelsen also reflected on the large quantity of typical food allergens that need to be consumed to provide 4 g of protein. This can be quite a considerable amount for infants, particularly given that they have other dietary requirements to fulfil, including the consumption of fruits, vegetables, and cereals. Mikkelsen emphasised the importance of practical nutritional counselling, and highlighted that dieticians can not only help with the prevention and management of food allergies, but can also help families when the allergy is outgrown. For example, many families continue to avoid certain foods despite clinicians’ advice to reintroduce these foods. Although there can be a reluctance to introduce food allergens into the diets of infants and children, Mikkelsen highlighted that this could be overcome. A good example of encouraging early introduction can be seen in the EarlyNuts study,23 which reported that peanuts and eggs were introduced into the diet of over 80% of 12-month-old children after the Australian infant feeding guidelines were updated in 2017.23
It was noted that although a vegetarian diet can provide sufficient nutrition and diet diversity, parents do require a considerable amount of knowledge and planning to ensure that children receive a well-balanced vegetarian or vegan diet.24 Mikkelsen highlighted that growing children have much higher nutritional needs than adults and that an inflammatory state can also lead to higher energy and nutrient needs. She emphasised that in any avoidance diet, it is important to focus on what is being substituted for the avoided foods. Although it is easy to obtain advice on what foods to avoid, there is a lack of available information on what to substitute these with. It is also important to ensure that families are aware that there are additional benefits to a diverse diet for the prevention and/or management of many current diseases, including cardiovascular disease,25 obesity,26 Type 2 diabetes,27,28 certain types of cancer,29,30 and infectious diseases.31 Mikkelsen concluded that as we now enter the era of encouraging a broad diet for infants, it is more important than ever to provide nutritional support and guidance to their families.
Early Introduction of a Multi-allergen Mixture for Prevention of Food Allergy: A Pilot Study
Kari Nadeau
Nadeau began by emphasising the importance of ensuring that children receive a wide variety of proteins, vitamins, and textures in their diet so that they can live without fear of eating. She also highlighted the increasing body of evidence that feeding a diverse range of multiple common food allergens early and frequently may reduce the risk of developing food allergies.3,11,32 In particular, it was noted that larger quantities of food allergens may not be required, with a recent study by Nishimura et al.33 demonstrating that consumption of small amounts of allergens can be sufficient to reduce the incidence of food allergies. Despite this growing evidence and recent guideline changes, the introduction of multiple food allergens to infants can be difficult to manage and there is a clear, unmet need to develop methods for introducing potential dietary food allergens that are both tolerable to children and convenient and practical for caregivers.
In this presentation, Nadeau described the results from a 1-year randomised, unblinded, prospective, descriptive pilot study (NCT04828603),34 which was designed to evaluate the safety and efficacy of three methods for the early introduction of food allergens.35 The study enrolled infants between 2 and 12 months of age, with approximately 50% of infants considered to be at high risk of atopy (defined as having either one first-degree relative with a food allergy or atopic dermatitis or two first-degree relatives with atopic disease). Infants with any chronic disease, any known genetic disease, or a known food allergy were excluded. Of the 180 infants enrolled, 51% were female and the median age was 6 months. The majority (51%) were Caucasian, with the remainder being either Asian (20%), African American (14%), or Hispanic (11%). Most participants (83%) had eczema, with 46%, 25%, and 12% described as having mild, moderate, or severe eczema, respectively.
The participants were randomised into 10 groups with stratification according to the risk of atopy. Participants received either a single food allergen (egg, milk, or peanut; n=15 each), a double food allergen (peanut and milk, peanut and egg, or milk and egg; n=15 each), or a 10-allergen mixture (comprising almonds, cashew, egg, hazelnut, milk, peanut, salmon, shrimp, walnut, and wheat) daily for 1 year. The 10-allergen mixture was given at either a low (300 mg/day; 30 mg of each allergen; n=15), medium (900 mg/day; 90 mg of each allergen; n=15), or high (3,000 mg/day; 300 mg of each allergen; n=15) serving size. The final group included 45 age- and sex-matched controls who avoided all potentially allergenic foods for the duration of the study. No increases in allergic reactions were reported regardless of risk stratification and baseline eczema status following the introduction of single, double, or a mixture of allergens, indicating that this approach is likely safe up to a serving size of 3,000 mg of protein.
Only half of the participants in the control arm (52%) passed an oral food challenge that was performed 2–4 years after the start of the study. In contrast, almost all participants (93–100%) who received the 10-allergen mixture passed (realitve quality factor: <0.01 for all dose groups versus controls; Figure 1). No significant differences compared with the control group were observed for any of the single or double food allergen subgroups.
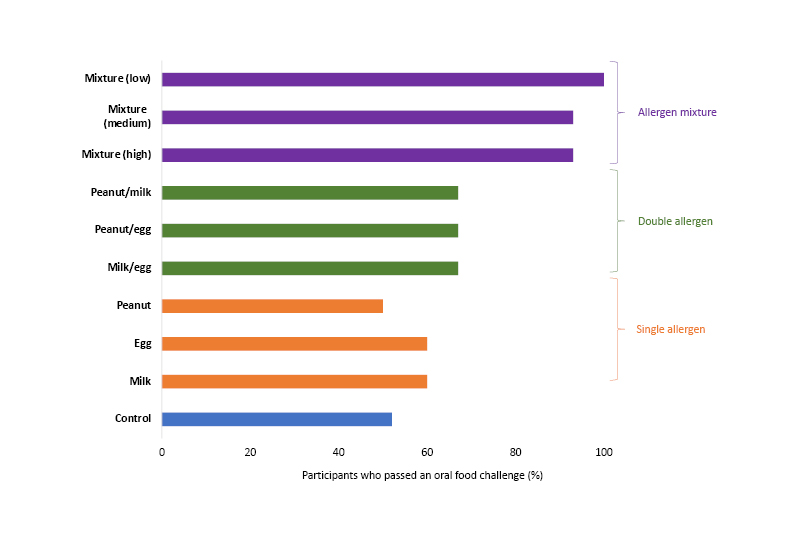
Figure 1: Results of an oral food challenge in infants who received a single, double, or 10-allergen mixture of food allergens for 1 year.
A food diary completed at the end of the study revealed that only 16% of infants in the control group were eating more than 10 foods as table foods by 1 year of age. This increased to 53–67% and 53–73% for infants who received one and two food allergens, respectively, and 93–100% for infants who received the 10-allergen mixture (Figure 2).
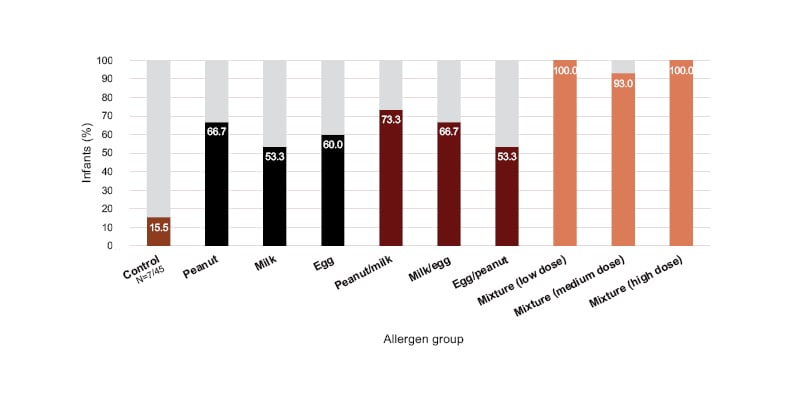
Figure 2: Percentage of infants eating more than 10 food items as table foods by 1 year of age.
Allergen-specific IgE and IgG4 levels were measured in blood samples collected from all participants at baseline and the end of the study. Peanut-specific IgG4/IgE ratios increased from baseline to the end of the study for all groups who consumed peanut allergen during the study, but not in the groups that did not consume peanut (Figure 3). Of note, a greater increase in this ratio was observed in the participants who consumed the 10-allergen mixture compared with those who consumed peanut allergen in the single or double allergen groups. Nadeau explained that this might indicate that the immunological response to a larger mixture of proteins may differ from that achieved with only one or two allergens. However, the reasons for this are currently unclear. Similar results were also observed for the milk-specific and cashew-specific IgG4/IgE ratios, with specificity only demonstrated for milk and cashew-containing foods, respectively. Nadeau also drew attention to the results from the control group. For these participants, the IgG4/IgE ratio is not stable but instead declines over time. Similar results have been observed in the control groups of other early introduction randomised controlled trials, indicating that the risk of developing food allergies may increase for infants who are not engaged with the early introduction of food allergens. It is also interesting to note that the increases in the IgG4/IgE ratio are driven by increases in IgG4 rather than decreases in IgE. This may be indicative of an increase in tolerance to these foods.
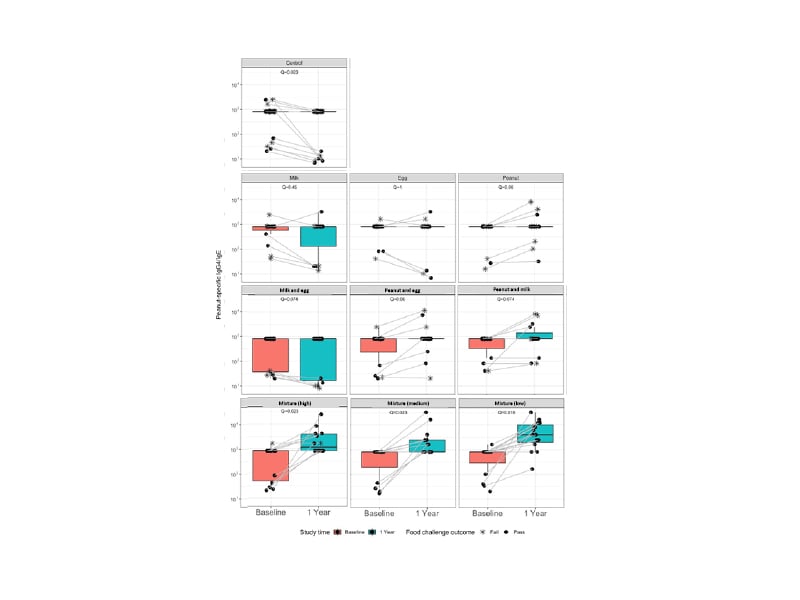
Figure 3: Peanut-specific IgG4/IgE ratios for the control, single, double, and multi-allergen groups at baseline and after one year from the start of the study.
Adapted from Quake et al.35
Nadeau concluded that there was an improvement in food tolerance across all active groups, with the percentage of participants able to consume up to 8 g of protein being significantly higher in groups receiving a 10-allergen mixture, regardless of serving size, compared with a control group. Consistent with this, biomarker analysis identified a trend for immune protection and loss of allergic mechanisms in patients receiving the 10-allergen mixture. Importantly, there was no increase in the incidence of eczema, even in patients who had eczema at enrolment or who were considered at high risk of atopy. In addition, infants who received the 10-allergen mixture were more likely to diversify their diet compared with participants in the control group or those receiving single or double allergens.
In summary, the results of this pilot study indicate that daily dosing of a small number of mixed food proteins may be a convenient, safe, and effective method for preventing food allergies in infants. Further research will be required to determine the serving size, frequency, and time to start early infant feeding that optimises tolerability and reduction in allergy risk. This should ideally be performed using randomised, prospective studies rather than performing retrospective, sensitivity analyses that extrapolate backwards using statistical modelling.
Early Data from the INTENT Study: Evaluating a Daily Multi-allergen Mixture
Wendy Sue Swanson
Swanson began by outlining how infant feeding guidelines have dramatically changed following the publication of the LEAP and EAT trial studies in 2015 and 2016, respectively.3,31 In 2017, the American Academy of Pediatrics (AAP) reversed its earlier guidance and endorsed the recommendations from the National Institute of Allergy and Infectious Diseases (NIAID) for an early introduction of peanut protein for infants who are at increased risk of developing a peanut allergy.36 In the same year, the U.S. Food and Drug Administration (FDA) released a qualified health claim stating that introducing food containing ground peanuts to infants with severe eczema and/or an egg allergy may reduce the risk of developing a peanut allergy by 5 years of age.37 In 2020, the American Academy of Allergy, Asthma and Immunology (AAAAI); the American College of Allergy, Asthma and Immunology (ACAAI); and the Canadian Society of Allergy and Clinical Immunology (CSACI) produced consensus guidelines stating that infants should be fed all common allergens from 4–6 months of age when solids are introduced.4 Subsequently, recent research has demonstrated that consuming even small amounts of multiple food allergens may be an effective strategy to lower the risk of food allergy development.33,35
Swanson proceeded to describe the development of the SpoonfulONE (Nestlé, Vevey, Switzerland) product, which contains a blend of 16 common allergens (30 mg each of almond, cashew, cod, egg, pecan, pistachio, salmon, sesame, hazelnut, milk, oat, peanut, shrimp, soy, walnut, and wheat). This product was developed based on findings from a 1-year feeding study in infants and children, which showed that consumption of a protein mixture with 10 foods was superior to consumption of single or double allergens at reducing levels of food-specific IgE, with 30 mg and 300 mg portions sizes being similarly effective.38 The safety of this product was evaluated in the I’m Eating Study (NCT03667118),39 which randomised 705 healthy infants aged 5–11 months to receive either the multi-allergen product or placebo daily for 28 days.40 Caregivers reported any symptoms that occurred in the 2 hours following consumption of the product or placebo each day. Overall, there was no significant difference between the product and placebo groups in the proportion of infants with any caregiver-reported symptoms.40
This product is currently being evaluated in the INTENT study (NCT04803981),41 a controlled, open-label, pragmatic, direct-to-participant trial that randomised healthy infants aged 4–6 months to receive either a standard diet alone or a standard diet plus a daily serving of the multi-allergen product. Infants with gastro-oesophageal reflux disease or with clinician-diagnosed food allergies were excluded. All data are caregiver-reported and collected directly from a mobile application platform (app). For the 18-month trial, all caregivers will complete monthly e-questionnaires on diet diversity and the age at which nine common foods (peanut, milk, cashew, egg, cod, shrimp, wheat, soy, and sesame) are introduced. For the active group, daily electronic online questionnaires to assess compliance and quarterly e-questionnaires on parental comfort with introducing food proteins and the convenience of long-term multi-food allergen product use will also be completed. The primary endpoint is the proportion of children able to tolerate five common foods (peanut, egg, cashew, cod, and sesame) in two feedings after 12 months in the study. Secondary endpoints include parental comfort with using this product and ease of use, diet diversity, and ability to tolerate nine common foods at 18 months. Safety will be evaluated via the collection of adverse events related to allergic reactions. Eczema and other symptoms of IgE-mediated allergic reactions will be considered adverse events of special interest.
Recruitment was potentially challenging due to the COVID-19 pandemic and the narrow window of eligibility in an infant’s life (eligibility was only when infants were between 4 and 6 months of age). However, online recruitment strategies have been successful, with approximately 75% of participants recruited via emails sent to parents who were members of the BabyCenter (San Francisco, California, USA) online parenting forum. Additional participants were recruited via social media or in-clinic advertisements. In total, 496 infants with eczema were recruited and stratified based on the severity of their eczema using the Patient-Oriented Eczema Measure (POEM) scale. A further 1,207 infants without eczema have also been recruited. The findings from the INTENT study may provide valuable information on the introduction of food allergens and serve as a blueprint for future large-scale, digital studies that evaluate strategies for the early introduction of infant food allergens.
Live Question and Answer Session
Angela Claver Monzón, Andrea Mikkelsen, and Wendy Sue Swanson
The session concluded with a live question and answer session. Monzón reiterated that many paediatricians and families still believe that certain foods should be avoided in infancy and that this belief may lead to ongoing problems with food allergies in the future. The key to improving this situation will be to teach appropriate strategies for early food introduction to healthcare professionals who work with children.
Swanson clarified that the patients in the control arm in INTENT are not receiving a placebo treatment but are real-world controls. They will continue to be followed up throughout the trial via the mobile app, with their caregivers continuing to complete surveys prompted by reminders sent through the app. Caregivers will also receive a small fee for completing each survey as motivation to participate. Both groups in the study will have access to standard educational resources via the app. Swanson also confirmed that the product used in INTENT only contains food items and not any other supplement or non-food items. She agreed that infants may benefit from exposure to allergens beyond food (e.g., pollen and mites) but that this can be achieved by taking babies outside. Swanson also stated that she would be happy to share the educational resources used for the INTENT app with other researchers, and that she will be presenting a video tutorial to demonstrate the function of the app at a separate European Academy of Allergy and Clinical Immunology (EAACI) session.
Monzón noted that differences exist between countries in their approach to preventing food allergies. She highlighted that it is important for all countries to inform parents and other paediatricians of the findings from recent studies. Swanson added that, in the USA, she is involved with events that provide information for new parents, including an update on the latest data that support early allergen feeding for infants. Educational sessions on infant feeding are also included at several academic meetings, including the AAP meeting. Information is also available in paediatric journals to ensure that paediatricians are aware of the most recent data that support the need for regular exposure to allergens to induce and maintain immunological tolerance.






