Abstract
Eosinophilic oesophagitis (EoE) is an increasingly prevalent chronic inflammatory disorder diagnosed by the presence of oesophageal symptoms and eosinophilic inflammation on endoscopic histology. Treatment of EoE centres around the ‘3 D’s’: drugs, diet, and dilation, which aim to both improve symptoms and prevent potential complications. Potential pharmacologic therapies include acid suppressing agents and corticosteroids, among others. Dietary strategies comprise the elemental diet, the empiric elimination diet, and the allergy testing-directed elimination diet. The therapeutic landscape of EoE is rapidly changing as our understanding of the disease evolves. This review aims to provide a comprehensive discussion of existing EoE therapies and to outline an approach to EoE management.
INTRODUCTION
Eosinophilic oesophagitis (EoE) is a chronic condition characterised by eosinophilic inflammatory changes of the oesophagus with associated oesophageal symptoms. It is a relatively new disease, first described in a 1978 case report of a patient with eosinophilic infiltration of the oesophagus.1 Since its discovery the number of cases has continued to increase, and currently the prevalence of EoE worldwide is estimated to be 0.5–1 cases in every 1,000 people.2 The disease is most frequently found among males of Caucasian descent.
Treatment of EoE focusses on symptom control and decreasing oesophageal inflammation. The mainstay treatment of EoE can be divided into three main categories: pharmacologic therapies which include both topical and systemic agents, dietary modification, and endoscopic interventions (Table 1). Choosing between these therapeutic options depends on several factors, including patient preference and clinical experience, among others. Certain treatment strategies such as the use of proton pump inhibitors (PPIs) are used early on as part of the initial diagnostic evaluation, while most endoscopic interventions, including endoscopic dilation, are reserved as second-line therapy.3 Dietary therapies range from the strictest elemental diet using an amino acid based formula, to the six-food elimination diet (SFED), and the tailored allergy testing-directed elimination diet. This review aims to provide a comprehensive discussion of existing EoE therapies and outline an approach to EoE management.
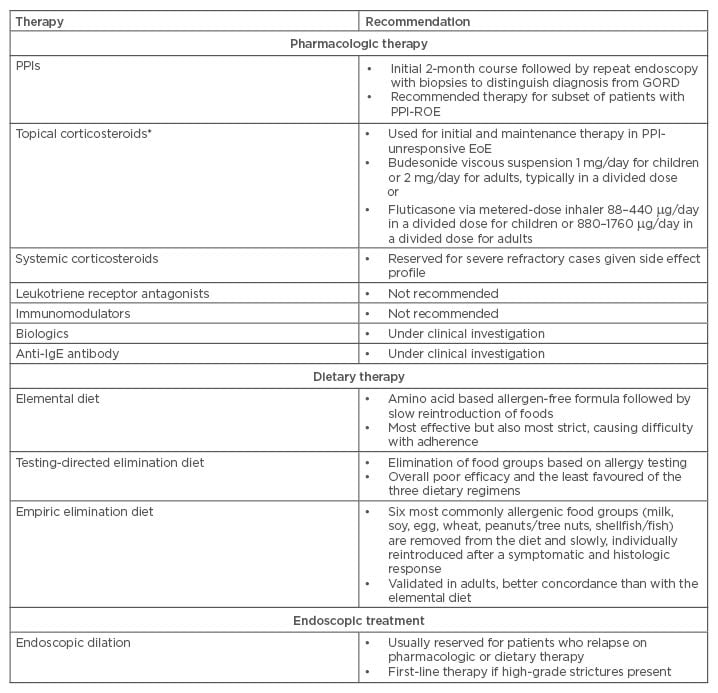
Table 1: Eosinophilic oesophagitis treatment summary.
GORD: gastro-oesophageal reflux disease; PPIs: proton pump inhibitors; IgE: immunoglobulin E; EoE: eosinophilic oesophagitis. PPI-ROE: PPI-responsive oesophageal eosinophilia.
*Note that no US Food and Drug Administration (FDA)-approved therapies for EoE have been approved to date. The dosing listed above is based on 2013 American College of Gastroenterology (ACG) published guidelines.
PHARMACOLOGIC THERAPY
Pharmacologic therapies in EoE aim to decrease oesophageal inflammation and alleviate symptoms. PPIs are initially recommended to help establish the diagnosis, but are not used as a sole treatment of EoE with the exception of the disease subtype PPI-responsive oesophageal eosinophilia (PPI-ROE). The mainstay of pharmacologic treatment for EoE involves corticosteroids, which several studies have confirmed to improve both oesophageal symptoms and histologic features of EoE. Histology has been a common metric of following patients during therapy, with the goal of achieving eosinophil reduction. Several newer pharmacologic options, such as the chemoattractant receptor homologous molecule expressed on T Helper type 2 cells (CRTh2) antagonist, have emerged in recent years with promising results, but remain experimental to date.4
Proton Pump Inhibitors
Patients with gastro-oesophageal reflux disease (GORD) are often found to have an elevated level of eosinophil infiltration in oesophageal tissue, a consequence of mucosal exposure to acidic contents and a presentation that can mimic EoE.5,6 During the diagnostic process, attempts to distinguish GORD from EoE are made by assessing responsiveness to PPIs. However, the benefits of PPI therapy appear to reach beyond their acid-suppressing effect. PPIs are thought to play an anti-inflammatory role through several mechanisms, including inhibiting cytokine production from the oesophageal epithelium, impairing neutrophil phagocytosis, and exhibiting anti-oxidant effects.7,8
Recent studies have revealed a subtype of EoE known as PPI-responsive oesophageal eosinophilia, defined by the attainment of histologic remission with the use of PPIs.9,10 Recognising this phenomenon, the latest guidelines recommend a 2-month PPI course followed by repeat endoscopy with biopsies to exclude PPI-ROE.11 In up to one-third of EoE patients who are found to have PPI-responsive disease, new data are emerging which supports long-term PPI therapy. A recent retrospective cohort study demonstrated that 73% of PPI-ROE patients remained in remission over a 12-month follow-up period and the majority of patients who relapsed re-achieved remission with PPI dose escalation, suggesting that PPI-ROE patients may require maintenance PPI therapy.12
Topical Corticosteroids
Topical corticosteroids are used as first-line agents and remain the mainstay of therapy in PPI-unresponsive EoE.13-15 Studies have found reduced oesophageal lamina propria remodelling and tissue fibrosis with topical steroid use.16,17 Fluticasone and budesonide are the two most commonly used topical agents in EoE with the largest amount of supporting evidence.18-28
In children, oral budesonide significantly improved symptom, endoscopy, and histology scores compared with placebo.19 In the adult population with active EoE, budesonide was found to induce clinical and histological remission.20 Long-term budesonide use has also been found to maintain clinical and histologic remission while being well-tolerated by patients in a 50-week trial.29 Recommended doses of budesonide administered as a viscous suspension are 1 mg daily for children <10 years old and 2 mg daily for older children and adults.14
Fluticasone is dispensed through a metered-dose inhaler into the mouth and then swallowed, at 88–440 mg 2 to 4-times daily to children and 440–880 mg twice daily to adults.14 In one of the earliest trials comparing fluticasone with placebo in paediatric patients, fluticasone induced remission in 50% of study participants compared with just 9% in the placebo group.24 A more recent multicentre trial indicated that 65% of patients receiving fluticasone achieved complete remission after 3 months.25 Interestingly, a systematic review and meta-analysis comparing five randomised controlled trials (RCTs) indicated that while topical steroids provoke histological remission, they may not significantly improve clinical symptoms.30 Overall, >40% of patients have been found to have steroid refractory EoE and 91% of patients experienced a recurrence of symptoms 9 months after completing treatment, prompting the question of the need for alternative therapeutic agents.30,31
The most commonly cited side effect of topical corticosteroids is oral and oesophageal candidiasis, although patients may also be at a rare risk of adrenal axis suppression and reduction in bone density.32 Based on these findings, the length of steroid treatment should be decided on a case-by-case basis with close monitoring for side effects.
Systemic Corticosteroids
The use of systemic corticosteroids to treat EoE is limited given their expanded side effect profile and analogous benefits compared with topical agents. In the one existing RCT comparing oral prednisone to topical fluticasone, histological and clinical improvement was seen in both groups after 4 weeks of treatment and there was no significant difference between the groups in time-to-relapse.22 However, 40% of patients on prednisone experienced adverse effects of the medication, including weight gain and Cushingoid features, compared with only 15% of fluticasone patients who developed oesophageal candidiasis and no systemic effects. With this in mind, in practice systemic steroids have been reserved for severe refractory cases of EoE, and when used are administered at high doses, for example prednisone at 1-2 mg/kg.11
Leukotriene Antagonists, Biologics, and Other Therapies
Montelukast, a leukotriene D4 receptor antagonist, has been studied as a potential therapeutic target in EoE, since leukotriene D4 is a chemotactic factor for eosinophils and could play a role in the disease pathogenesis.15 One small prospective study of 12 patients demonstrated a symptomatic but not histologic benefit, and noted the occurrence of multiple side effects.33 Furthermore, when administered to patients in remission who completed a 6-month course of fluticasone treatment, montelukast did not succeed in maintaining clinical or histological remission.34 Based on this evidence, the use of leukotriene receptor antagonists is not a recommended treatment regimen.
Multiple biologics have been studied in EoE, with the most researched being interleukin (IL)-5 antibodies. Cytokine IL-5 is a key eosinophil growth and activation factor.35 Mepolizumab, a humanised monoclonal antibody against IL-5, was studied in two small RCTs of adult and paediatric EoE patients.36,37 Both trials demonstrated a significant reduction in oesophageal eosinophilia in the treatment group, and while the drug was well-tolerated in the adult population there was no evidence of symptomatic improvement. In a RCT in the paediatric population of reslizumab, an IL-5 neutralising antibody, there was a statistically significant decline in eosinophil numbers after treatment, but no significant improvement in symptoms compared with placebo.38 The use of the tumour necrosis factor alpha inhibitor, infliximab, was described in three patients of refractory EoE, determining no reduction in either eosinophilic infiltration or symptom scores.39 To date, biologic therapies are currently under study and are not recommended for routine use. There are currently several ongoing trials in EoE, including an advanced examination of the role of infliximab in EoE, as well as studies investigating novel agents such as the study of dupilumab, a fully human monoclonal antibody directed against IL-4 receptors.
Very limited data exists regarding the use of immunomodulators to treat EoE. A case series of three patients treated with azathioprine or 6-mercaptopurine resulted in all three patients experiencing clinical and histological remission.40 However, given the significant side effect profile of these agents with little accompanying data on their benefit, these agents need to be studied more extensively before they become recommended therapy.
Omalizumab, an anti-immunoglobulin E antibody, is one potential therapeutic target in EoE. A case report of two patients noted improvement in symptoms after omalizumab treatment; however, no histologic change was found.41 Additionally, no RCTs exist involving omalizumab in the current literature. Furthermore, given that EoE is a transmural disease, new fibroblast-targeted therapies, such as JAK-STAT6 pathway inhibitors, are currently under investigation with the potential of treating both the epithelial fibrosis and the subepithelial fibrosis.42 Finally, OC000459 is a receptor antagonist to the CRTh2 which mediates chemotaxis of inflammatory cells, including eosinophils. This agent has been more extensively studied in asthmatic patients; however, a RCT of 26 patients demonstrated a moderate but significant improvement in oesophageal eosinophilia and its symptoms after 8 weeks.43
DIETARY THERAPY
EoE is strongly associated with multiple allergic conditions, including atopic dermatitis, asthma, and food allergies. The avoidance of potential food allergens through dietary modification in patients with EoE has been used as a non-pharmacologic treatment strategy. Patients are most effectively managed by a multi-disciplinary team including allergy specialists and registered dieticians to help implement and oversee dietary therapy. Three distinct dietary approaches have been studied in the treatment of EoE: elemental diet, testing-directed elimination diet, and the empiric elimination diet.
Elemental Diet
Patients on the elemental diet are placed on an amino acid based allergen-free formula. The elemental diet has been found to be the most effective of all diets in EoE, with 90.8% of patients achieving histologic remission.44 The diet’s efficacy has been most extensively studied in children, demonstrating a significant improvement in both symptoms and histologic findings.45,46 Only one study in the existing literature verified the benefit of the elemental diet in adults, with complete or near complete endoscopic response in 72% of patients.47 Beyond decreasing eosinophil infiltration, there may be evidence that the diet also decreases the degree of oesophageal fibrosis.48
Once patients achieve clinical and histological improvement, foods are slowly introduced starting with the least allergenic. The main disadvantage of this regimen lies in its strict guidelines, avoidance of solid food, and overall poor tolerability particularly in the adult population. Consequently, practitioners often reserve it for patients who fail other therapies.
Testing-Directed Elimination Diet
Allergy testing-directed elimination diets are based on the results of allergy testing from radioallergosorbent or skin-prick tests. In a large study of the paediatric population, 49% of participants had normalisation of their oesophageal biopsy findings, after the exclusion of participants treated with an elemental diet.49 The largest prospective study of adults utilising an individualised diet based on three different skin tests demonstrated a poor response, with 66% of patients having neither a clinical nor histological response to the dietary therapy.50 A meta-analysis of 14 studies, including both paediatric and adult patients, found the diet’s overall efficacy to be 45.5%.43
One limitation of this approach lies in the origin of the diet design, as skin prick tests are only successful in identifying foods causing allergic symptoms in approximately 13% of cases.51 Combined with its suboptimal results compared to the other available dietary therapies, the testing-directed elimination diet has fallen out of favour for non-paediatric patients in general practice.
Empiric Elimination Diet
Compared with allergy testing, the best way to identify food allergens has been confirmed to be through food reintroduction.51 The most extensively studied empiric elimination diet is the SFED where the six most commonly allergenic food groups (milk, soy, egg, wheat, peanuts/tree nuts, and shellfish/fish) are removed from the patient’s diet. To date, extending this diet to avoid rye and barley, or implementing a gluten-free diet, has not been proven to be effective in improving clinical or histologic outcomes in EoE.52 After confirmed symptomatic and histologic response, on average 6 weeks later the food groups are slowly and individually reintroduced while monitoring for disease reoccurrence. An individualised approach to food reintroduction can be applied given no existing guidelines outlining the order in which food can be reintroduced. However, it should be noted that milk and wheat products are usually added last, as they are the most common source for allergies.53
The SFED was first studied in children, where 74% of the study participants experienced improvement of oesophageal inflammation.54 Subsequent studies validated comparable findings in adults, with the most common food triggers identified as milk and wheat.51,55 A group investigating the four-food elimination diet (FFED) found its effectiveness to be 54%, with a total of 72% of patients improving if the SFED was applied to the FFED non-responders.56 A recognised advantage of the empiric elimination diet is significantly improved concordance compared to the elemental diet. Additionally, once specific food allergens are identified, long-term avoidance of the offending agents has shown to produce both clinical and histopathological remission.55
ENDOSCOPIC TREATMENT
Endoscopic dilation is usually reserved for patients who relapse on pharmacologic or dietary regimens, and occasionally as first-line therapy if high-grade strictures are initially present. Dilation is performed using one of three types of dilators: mercury or tungsten-filled bougies, wire-guided polyvinyl dilators, or endoscopically-guided balloon dilators.57 The safety of these procedures has been verified with rare incidence of major complications.58 A meta-analysis of RCTs of dilations in EoE confirmed that perforation rates remain <1%.59,60 Less threatening complications such as post-procedural chest pain can occur in up to 75% of patients, but have been found to be well-tolerated by patients.61
Retrospective studies have shown that dilation success, defined by symptom resolution, occurs in 83–93% of patients.61,62 In the absence of severe oesophageal stricturing disease, it is reasonable to consider initial treatment with medical or dietary therapy before proceeding to dilation.14 A RCT of newly diagnosed EoE patients with mild-to-moderate strictures on PPIs and fluticasone, randomised to dilation or no dilation at time of endoscopy, demonstrated no significant reduction in dysphagia scores between the treatment groups, highlighting that patients without severe strictures may not benefit from endoscopic therapy.63
Given frequent recurrence of symptoms post-dilation in 20–25% of patients, serial dilations are often performed to achieve a symptom response.59,64 In a retrospective study of steroid-naïve EoE patients, the authors used endoscopic dilation as a long-term therapy and maintained patients’ symptoms over 13 years with biennial dilations.65 Sequential dilations are necessary as the intervention does not itself inhibit the pathophysiology of the disease and does not prevent future stricture formation. Studies showed that dilation did not influence the histologic presentation of EoE, with no reduction in oesophageal eosinophilia after the procedure.61,66 Thus, when choosing a therapy, it is important to note that oesophageal dilation is thought to treat the clinical symptoms of EoE without changing the course of the underlying disease process.
CONCLUSION
EoE has been defined as a clinicopathological diagnosis, incorporating assessment of eosinophilic burden on histology and patient symptoms and presentation.14 EoE treatment seeks to target the underlying inflammatory process and suspected triggers, such as food allergens. In the treatment of EoE, a multidisciplinary and collaborative care team comprising of gastroenterologists, allergy/immunology specialists, and dieticians is vital to a successful treatment of comorbid allergic diatheses. Ultimately the goal of treatment is to improve patients’ quality of life by reducing symptoms and preventing complications. Current therapeutic options suggest initiating a course of PPIs to confirm the diagnosis and using topical corticosteroids as the mainstay of treatment (Figure 1). The majority of research in dietary therapies has been conducted in the paediatric population, and more data is needed to confirm their efficacy in adults. Future therapies will be based on an improved understanding of the disease pathogenesis, and treatment recommendations will continue to evolve with ongoing research and the development of novel promising therapies.
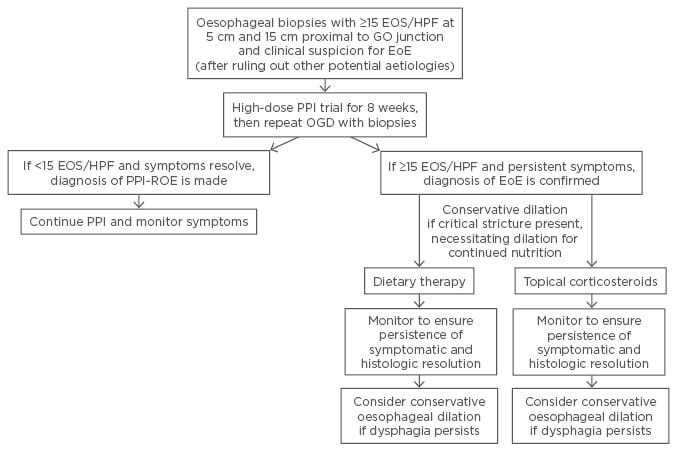
Figure 1: Algorithm for diagnosis and management of eosinophilic oesophagitis.
GO: gastro-oesophageal; EOS/HPF: eosinophils per high-power field; PPI: proton pump inhibitor; EoE: eosinophilic oesophagitis; OGD: oesophagogastroduodenoscopy.
Modified from Kavitt RT et al. with permission.67






