Abstract
Thymoma is often associated with many other autoimmune disorders and clinical conditions. Good syndrome is one of the rare associations between thymoma and immune deficiency that occurs in both males and females in the 4th or 5th decade of life. Patients can present with various invasive encapsulated organisms, and opportunistic viral and fungal infections, due to immune defects. The authors report a case of a 57-year-old male with underlying thymoma and lichen planus which were diagnosed 3 years apart. He presented with atypical pneumonia during the COVID-19 pandemic, and was treated for multiple atypical infections, including cytomegalovirus and Pneumocystis carinii pneumonia. His immunological investigation panel revealed low IgA and IgG levels with normal IgM levels, and an overall deficient total B cell count. His CD4:CD8 ratio was reversed at 0.28. The patient recovered well after Ig replacement therapy once the Good syndrome diagnosis was made. The authors highlight the importance of a high index of clinical suspicion in dealing with this uncommon concomitant occurrence of Good syndrome during the COVID-19 pandemic for a swift and timely diagnosis and management. Immunological investigation panels, including T cell subsets, B cells, and quantitative Ig levels, should be considered routinely in patients with underlying thymoma presenting with opportunistic infections.
Key Points
1. Even during a pandemic, physicians should remain vigilant for rare immunodeficiencies such as Good syndrome, in patients experiencing refractory severe infections.
2. Ig levels should be assessed in patients with thymoma who present with unresolved infections.
3. Intravenous Ig replacement therapy is effective in treating Good syndrome.
INTRODUCTION
Thymoma-associated Good syndrome (GS) is a rare and intriguing clinical entity. It is characterised by the coexistence of thymoma and hypogammaglobulinaemia, leading to immune system dysfunction and invasive infections due to encapsulated bacteria, fungi, and viruses.1 It commonly occurs in both male and female adults in their 40s–60s.2 Many patients with GS also may have lichen planus involvement, which some authors have even included in the clinical features of GS. This is a case of a male patient with underlying thymoma, who was also receiving treatment for lichen planus, and presented during the COVID-19 pandemic with multiple opportunistic infections leading to the diagnosis of GS. He was successfully treated with intravenous (IV) Ig. The authors intend to highlight the diagnostic dilemma of atypical infections that could mimic COVID-19, which reminds us to have a high index of suspicion, and investigate thoroughly when encountering such atypical infections with poor response to antimicrobial treatment, more so in the background of underlying thymoma.
CASE REPORT
The patient is a 57-year-old male with an underlying prolapsed disc and thymoma. He was found to have a thymoma 4 years before presentation, after an incidental finding of widened mediastinum on a chest radiograph, done pre-operatively for a left elbow abscess and cellulitis. A contrast-enhanced CT thorax done back then showed a large well-defined solid mass at the right lower anterior mediastinum measuring approximately 8.5×9.0x9.3 cm. A CT-guided biopsy reported thymoma type B2, but he defaulted on the subsequent follow-up. He also had a history of lichen planus involving oral mucosa and bilateral upper and lower limbs since approximately 7 months, and was on oral azathioprine 150 mg daily, as well as multiple courses of oral prednisolone.
He presented to the authors with a 1 month history of shortness of breath, and reduced effort tolerance associated with fever. He was tachypnoeic with Type 1 respiratory failure. The oxygen saturation was 78% on room air. White cell count was raised at 11.1×109 /L (reference range [RR]: 4.5–11.0×109 /L), c-reactive protein was 99.8 mg/dL (RR: 0.3–1.0 mg/dL), and erythrocytes sedimentation rate was 81 mm/h (RR: <15 mm/h). Both serum albumin and globulin levels were low at 29 g/L (RR: 35–50 g/L) and 19 g/L (RR: 23–34 g/L), respectively. The chest radiograph showed consolidation in both lung fields (Figure 1). Urgent CT thorax-abdomen-pelvis showed features of organising pneumonia with thymoma 6.9×4.8×7.6 cm, and no evidence of pulmonary embolism. He was treated for healthcare-associated pneumonia as he was admitted to another hospital for a prolapsed disc just 5 days prior. IV piperacillin/tazobactam was started along with IV dexamethasone due to suspicion of COVID-19 pneumonia. However, he continued to further deteriorate, needing invasive ventilation with PaO2/FiO2 ratio of 300. His nasopharyngeal swab for SARS-CoV-2 PCR was repeatedly negative three times.
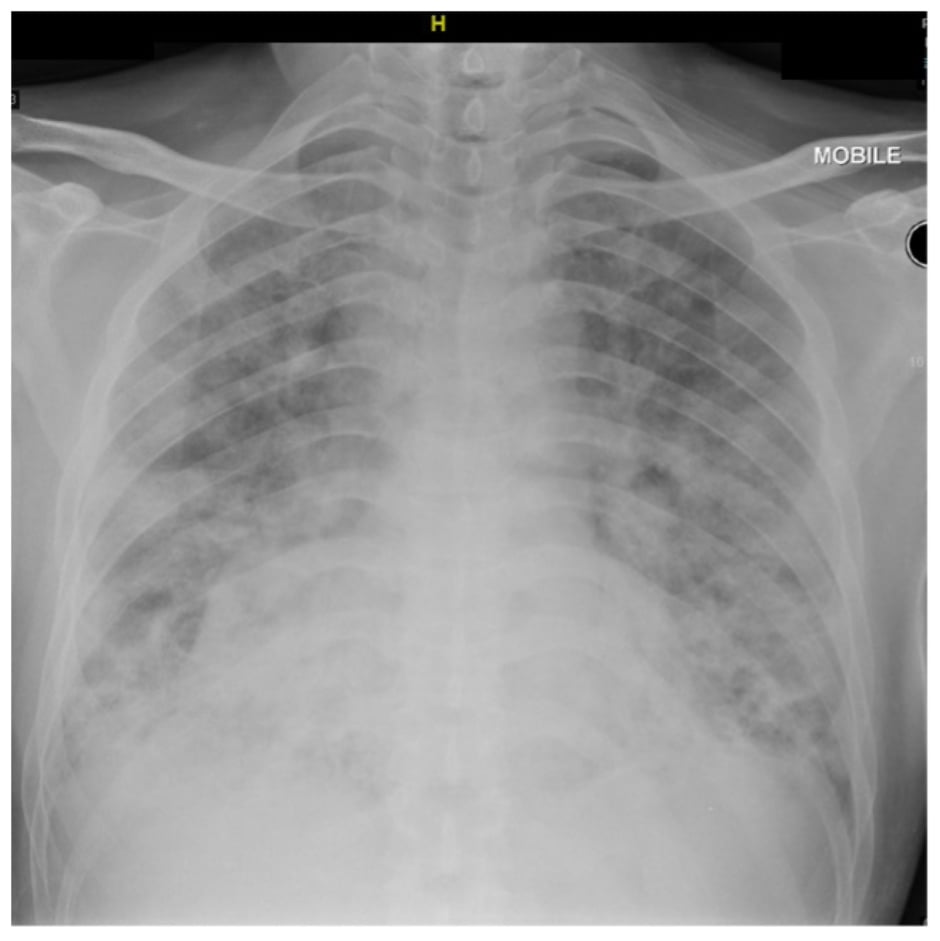
Figure 1: Plain chest radiograph upon admission showing consolidation in both lung fields.
He remained critically ill with persistent respiratory failure despite treatment escalation with IV meropenem, and additional IV amphotericin B and oral oseltamivir. Tracheal aspirate for respiratory infection panels was positive for Pneumocystis carinii pneumonia (PCP) DNA, and his serum cytomegalovirus (CMV) PCR was also positive. Serum anti-HIV antibody, hepatitis B surface antigen, and anti-hepatitis C virus antibodies taken were all negative, and the tracheal aspirate for tuberculosis GeneXpert turned out to be negative. IV trimethoprim-sulfamethoxazole and IV ganciclovir were initiated. Given the presence of opportunistic infections with low globulin levels, a diagnosis of non-HIV-related secondary immunodeficiency was suspected.
His acetylcholine receptor antibody was found to be positive at 1.29 nmol/L (RR: <0.4 nmol/L). Serum Ig results came back with normal levels of IgM at 1.41 g/L (RR: 0.4–2.3 g/L), and low IgA and IgG at 0.59 g/L (RR: 0.7–4.0 g/L) and 1.03 g/L (RR: 7.0–16.0 g/L), respectively (Table 1). Both complement levels C3 at 0.77 g/L (RR: 0.5–0.9 g/L) and C4 at 0.14 g/L (RR: 0.1–0.4 g/L) were normal. T and B cell panels enumeration showed reduced B cells, Th cells, natural killer cells, and a low CD4:CD8 ratio (Table 2). Diagnosis of GS with subclinical myasthenia gravis (MG) was subsequently made. A cycle of IV Ig 0.4 g/kg was initiated.
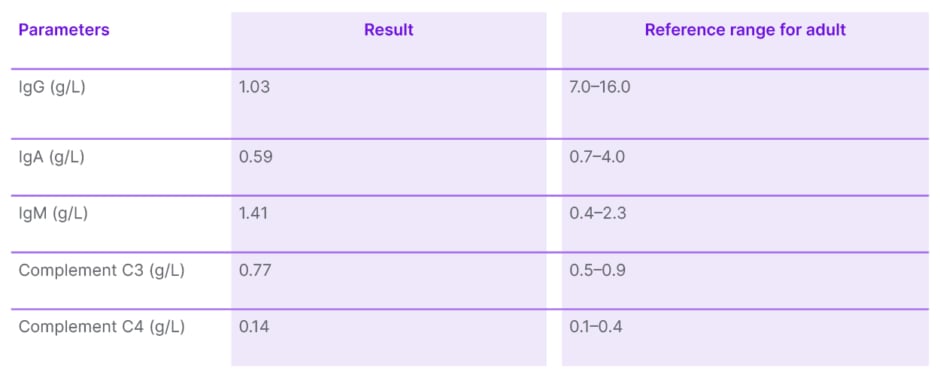
Table 1: Immunoglobulin, complement C3, and C4 level.
Adapted from de Vries et al.3
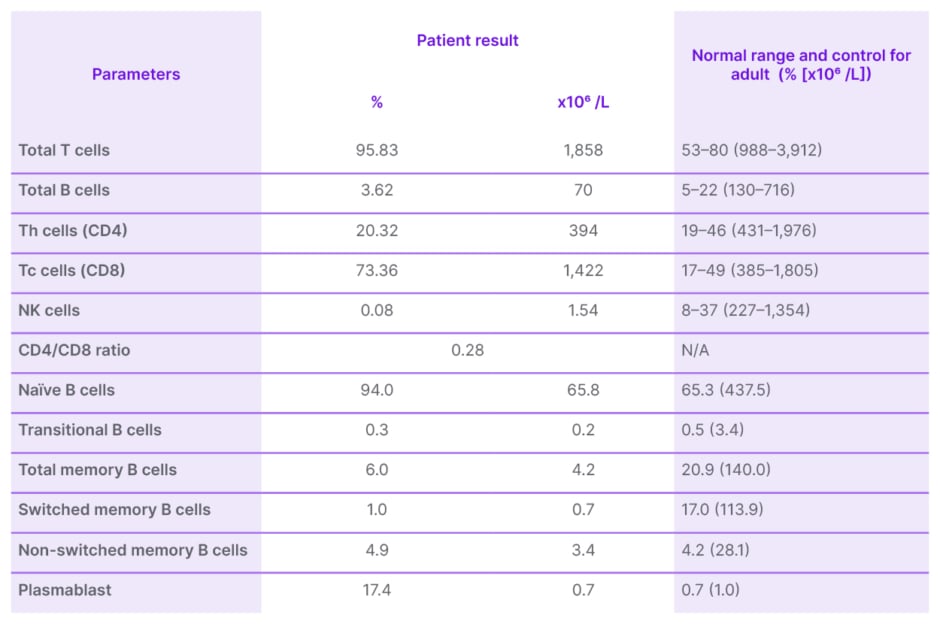
Table 2: T and B cell panels enumeration.
NK: natural killer; Th: T helper.
The prolonged intensive care unit stay was also complicated by upper gastrointestinal bleeding, pneumothorax secondary to barotrauma, Acinetobacter modestus, and extended spectrum β-lactamase Klebsiella pneumonia infection. He had undergone oesophagogastroduodenoscopy, tracheostomy, chest tube insertion, and treatment with high dose IV ampicillin/sulbactam for his intensive care unit stay-related complications.
Following the treatment with IV Ig, he was able to be weaned off invasive ventilation to tracheostomy mask O2 10 L/min supplementation. He then requested a transfer against medical advice to another healthcare facility for rehabilitation.
Although the presentation of this patient is typical of GS, he presented to the hospital during the COVID-19 pandemic. Consequently, he was treated with immunosuppressants such as dexamethasone, and underwent repeated nasopharyngeal swabs for SARS-CoV-2 PCR testing before any suspicion of opportunistic lung infection could be raised, ultimately leading to the diagnosis of GS.
DISCUSSION
GS was first reported by Good et al.4 in 1954, describing an association between thymoma and hypogammaglobulinaemia. The incidence of hypogammaglobulinaemia in patients with thymoma was approximately 6–11%. Most cases of GS are adult-onset affecting people from 40–60 years old, with a prevalence of one per 700,000 adults.2 The immunodeficiency may precede thymoma discovery. Some literature reported that the full syndrome is generally established within 6 years of the first presentation, characterised by severe or chronic infections, with or without autoimmune manifestations.1 This is consistent with the authors’ case, where the patient was diagnosed with thymoma 4 years prior, found out to have lichen planus 3 years later, and subsequently presented with severe opportunistic infections leading to the diagnosis of GS.
Clinical Features
The diagnosis of GS is challenging and requires a high index of suspicion in combination with immunological testing. Similar to X-linked agammaglobulinaemia and common variable immune deficiency (CVID), GS commonly presents with recurrent sinopulmonary infection secondary to encapsulated organisms.1 GS was previously thought to be a subset of CVID with thymoma. However, in contrast with X-linked agammaglobulinaemia and CVID, opportunistic infections caused by CMV, mucocutaneous candidiasis, varicella zoster, human herpesvirus, and P. carinii have also been seen in GS.1 This is consistent with the authors’ case whereby the patient had respiratory failure secondary to PCP, and possibly CMV pneumonia. Apart from respiratory illness (74%), Jansen et al.5 reported that 35% of the studied GS population had gastrointestinal infections, and 21% had skin and soft tissue infections, followed by 15% with urinary tract infections. With regard to causative micro-organisms, bacterial infections account for 80% of the cases, followed by viral (23%) and fungal infections (26%).5
GS has been associated with autoimmune conditions such as pure red cell aplasia (31.3%), MG (27.7%), and lichen planus in 51.2% of patients.6 Relating to this, the authors’ patient was being treated for lichen planus 1 year before the current encounter and was found to have subclinical MG during admission. The most common form of lichen planus found in those with GS was the erosive type concerning 89% of cases, followed by reticulated plaque and hyperkeratotic plaque in approximately 21% and 11% of cases, respectively.7 In certain cases, LP lesions may be improved or resolved after thymectomy.8
One interesting aspect of this case is that since the patient presented with severe pneumonia during the COVID-19 epidemic, the initial diagnosis of COVID-19 was at the top of the list. Multiple reports have emerged linking the complicity of COVID-19 in patients with GS.9,10 Some also highlighted the diagnostic dilemma in dealing with an immunocompromised patient, and severe pneumonia that could mimic COVID-19 during this period of epidemic. Tehrani et al.11 documented that their patient with CVID was empirically treated for COVID-19 but turned out positive for PCP.
Laboratory Findings
Marked hypogammaglobulinaemia with reduced or absent numbers of circulating B cells is the most prominent immunological finding in GS. A study found that almost 100% of patients with GS have low serum IgG levels, 86% have low IgA, and 92.6% have low IgM levels.6 In the authors’ case, the patient had low IgG and IgA with normal levels of IgM. Other important alterations in the immune profile of GS are the inversion of CD4/CD8 T cell ratio, and reduction of T cell mitogen proliferative responses. Interestingly, opportunistic infections like CMV infection and PCP can occur in the presence of significantly greater T cell numbers in the GS population.12 This was also seen in the authors’ patient, as his Th CD4 counts were 394 cells x106 /L, slightly lower than the normal range (432–1,976 cells x106 /L). This suggests functional T cell defects that are responsible for the development of these infections. The authors were unable to investigate further on lymphocyte proliferative response and specific antibody response to vaccination, as the patient decided to be discharged against medical advice.
Management and Prognosis
Complete surgical tumour resection with or without post-operative chemotherapy or radiotherapy reduces the risk of local invasion, and alleviates the associated autoimmune conditions. Nonetheless, the immunological irregularities are irreversible by the excision of the thymoma alone.6 This implies that hypogammaglobulinaemia stems from either an autoimmune or immunoregulatory process.13 Ig replacement therapy is used for antibody deficiency to control and prevent severe recurrent infections.
In terms of overall prognosis, median survival for patients with GS is 14 years with a mortality rate of 41%, which largely depends on the severity and type of complications.5 This mortality rate is undeniably higher than in CVID. However, note that the median age of GS diagnosis is much older, at 58 years old, compared to CVID, which is 30 years old for males and 33.5 years old for females.5 This large age gap in the presentation of the two conditions may also contribute to the overall prognosis.
CONCLUSION
Despite the COVID-19 epidemic, one should not only think about COVID-19, especially when the standard treatment does not show improvement. A high index of suspicion is pivotal in diagnosing GS, especially when there is a combination of thymoma and opportunistic infection. Serum IgG, M, and A can be performed routinely in patients with thymoma, with a more extensive study for those with opportunistic infections or other autoimmune associations. Ig replacement therapy is effective in treating immunodeficiency in GS.





