Allergic diseases affect approximately one-quarter to one-half of the average population under 50 years of age in Central Europe.1 Due to the high proportion of affected individuals, allergy testing needs to be performed on a large scale, with high sensitivity and specificity at a low cost. Skin tests are the most important diagnostic measure fulfilling these requirements: they can be performed immediately and, quite in contrast to laboratory tests, the results of skin prick tests for the diagnosis of immediate allergy (IgE-mediated: Type I) can be assessed, and discussed with the patients 15–20 minutes later. Patients do not need to be called in for a second appointment to discuss the results of serum-based determination of specific IgE. Recently, we demonstrated that the sensitivity of skin prick tests is superior to the measurement of allergen-specific IgE, even for modern molecular allergens.2 In T cell mediated allergy of the delayed type (contact dermatitis: Type IV), patch tests read after 48–72 hours are the only available diagnostic measure.
Although allergists worldwide recognise the immeasurable value of skin testing as the primary test for more than two-thirds of their patients in their daily work routine,3 we are faced with the critical situation of a gradual decline in commercially available test preparations. What are the reasons for this development? Allergy diagnosis is a niche market in which medical diagnostic companies generate little financial income.4 In an effort to improve the quality of diagnostic allergens, the European legislation on medicinal products 2001/83/EC and its refinement in 2007 recognised preparations for allergy skin testing as drugs.5 While quality and inter-batch reproducibility are laudable goals, European regulation authorities have forgotten that there is no market to compensate for the high economic burden caused by this legislation. Diagnostic test allergens must be registered in the same way as therapeutic allergens that are used as drugs. Once registered, registration must be maintained on a regular basis. If a registered test solution does not become introduced onto the market within a certain time frame, the registration is automatically lost after a few years due to a ‘sunset clause’.6
Reimbursement for allergy testing by the public healthcare system is very limited and, therefore, the diagnostic industry has not been able to pass on these new costs to allergists.7 Previously, diagnostics for Type I allergies were considered a by-product of allergen manufacturers, and revenue from the sales of allergen solutions for allergen-specific immunotherapy cross-funded diagnostics. Therefore, it was clear from the beginning that the first implementation of the European law into a national law, which occurred in the most important allergen market in Germany in 2008,8 risked to reduce the number of available solutions due to the high administrative costs. However, no one anticipated that the industry would drastically withdraw diagnostic allergens within the first few years of the new legislation coming into effect. Between 2011 and 2018, the number of approved skin prick test solutions for immediate-type allergies in Germany decreased from 522 to 378 (-27%) and for delayed-type allergies from 343 to 132 (-61%).9
In 2017 there was also a revision of the European ‘In-Vitro Diagnostic Device Regulation’ (IVDR 2017/746), which came into force on 26th May 2022, without the need for translation into national law.10 The reason for this was to prevent future medical fraud such as the Theranos scandal. Ultimately, this has the same consequences for niche allergology products as the legislation on in vivo diagnostics.11 Again, allergology is faced with the decline of in vitro tests for rare allergens. Consequently, the loss of in vivo testing cannot be replaced by switching to in vitro testing systems. This is especially problematic in the field of occupational medicine, where only a small number of patients are affected and subsequently tested.12
Skin testing is currently ‘under fire’. On the one hand, existing diagnostic allergens have been withdrawn from the market; on the other hand, hardly any new skin tests have come onto the market for two decades. Patch tests in particular are in a ‘frozen’ state, with the introduction of new allergens already dating back to the early 2000s.13
As a countermeasure, the European Academy of Allergy and Clinical Immunology (EAACI) has drawn up a position paper listing the most important requirements to European policy makers to maintain and hopefully even increase the availability of diagnostic allergens.14 The most important points include: simplification of the approval process for new diagnostic allergens; the homologous groups principle, meaning that not every single allergen from the same allergen family requires a full dossier for market authorisation; fee reduction of marketing authorities; and increased reimbursement schemes in national healthcare systems to compensate the increasing costs of skin testing (not only the diagnostic allergens, but also the recent enormous increase in labour costs for highly specialised and trained health personnel in allergology).
How could we get out of that situation? By going back to the way we did it a long time ago and making the skin prick test solutions ourselves again, without any standardisation? In a recent study, a Dutch group compared five ‘homemade’ extracts with commercially available food extracts from one of the largest allergen manufacturers still available at the time. They found mixed results with a good correlation for hazelnut and walnut extracts, but not for apple, peanut, and peach.15 Thus, ‘homemade’ extracts may not be the way to go.
Another approach could be to minimise skin prick test panels, since many standard panels test the major inhalant allergens twice, e.g., birch pollen plus hazelnut pollen from beech trees, or Dermatophagoides pteronyssinus plus Dermatophagoides farinae from the house dust mites. When applying the concept of cross-reactivity within homologous allergen families, it is sufficient to use only one representative of an allergen family.16
Not all is lost, yet. A new European Union (EU) guideline ‘Recommendation on common regulatory approaches for allergen products’ (Co-ordination group for Mutual recognition and Decentralised procedures – human [CMDh]/399/2019) has been implemented to overcome this situation.17 The new CMDh guideline may open a small window, as diagnostic allergens for in vivo skin testingwill be treated somewhat less stringently than allergen products to be used as therapeutics.17
Nevertheless, the following allergens require full marketing authorisation, as mentioned in Annex I of CMDh/399/2019, regardless of whether they are used for diagnostic or therapeutic purposes (Table 1). An originally included Annex II, which listed additional or rarer allergens, was deleted at the request of many experts during the public consultation on this guideline.
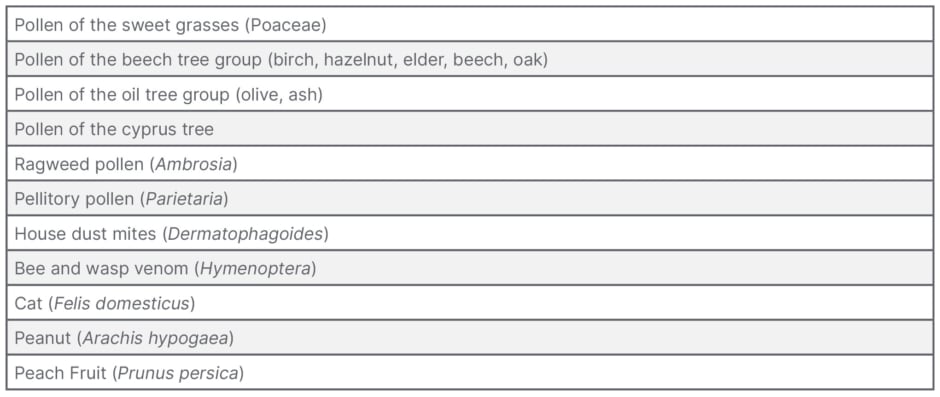
Table 1: Allergen sources for which full marketing authorisation is required according to Annex I of Co-ordination group for Mutual recognition and Decentralised procedures – human (CMDh)/399/2019.17
We can only hope that these changes will make a difference before the last available standardised skin test allergen for the diagnosis of immediate allergies (prick tests) and delayed allergies (patch tests) will have become ‘extinct’.





