Abstract
Introduction: Plasmacytoid dendritic cells (pDC) are unique antigen-presenting cells that may be implicated in allergic disease because they bind IgE on their surface and modulate important Th1/Th2 cytokine responses. The authors previously discovered that IgE is not readily removed from pDCs using omalizumab to the same extent observed for basophils, suggesting that a portion of the IgE on pDCs may be membrane bound.
Methods: A cross-sectional study was performed and recruited adult volunteers from Johns Hopkins Asthma & Allergy Clinic, Baltimore, Maryland, USA, between 2022–2023. Individuals with varying levels of serum IgE were selected, and those on subcutaneous immunotherapy or biologic therapy were excluded. Basophils and pDCs were isolated using established protocols. In order to isolate peripheral blood mononuclear cells, blood was also drawn from consenting donors with a wide range in total serum IgE levels. B cells and pDCs were identified by flow cytometry by gating on CD19/CD27 and blood dendritic cell antigen 2/CD123, respectively. Quilizumab, a mouse anti-human monoclonal IgG1 antibody with specificity only for membrane-bound IgE and surrogate for omalizumab, was used to detect this molecule in both cell types.
Results: When used in vitro at 1.5 mg/mL, omalizumab removed ~80–90% of the IgE expressed by basophils. In contrast, IgE expression decreased only 30–40% on similarly treated pDCs. There was no significant difference in the number of pDCs staining for membrane-bound IgE between the isotype control and quilizumab (P=0.125, 0.165). However, the proportion CD19+27+ B cells staining for quilizumab compared to other PBMC was 54:1 (P=0.015).
Conclusion: While pDCs express significant levels of surface-bound IgE, there is no appreciable amount of membrane-bound IgE noted. The reason for omalizumab’s poor ability to remove pDC-bound IgE compared to its effectiveness at removing circulating IgE remains unknown.
Key Points
1. Plasmacytoid dendritic cells (pDC) are a unique subset of antigen-presenting cells known to be involved in innate immunity, cancer, and immune tolerance.2. It is not clear why pDC express a variant of the IgE (FcεRI) receptor on their surface, or if they also exhibit a membrane-bound version of this receptor.
3. This study confirms that pDCs do express high levels of FcεRI that very tightly bind IgE on their surface, but no appreciable amount of membrane-bound IgE was detected.
INTRODUCTION
Plasmacytoid dendritic cells (pDC) primarily function as weak antigen-presenting cells (APC), but have also been shown to express functional receptors for key elements of both the innate and adaptive immune responses.1,2 pDCs induce the proliferation of naïve T cells towards Th1, Th2, or Th17 immune responses and have been implicated in allergic disease processes, most prominently in allergic asthma and atopic dermatitis.1-5 pDCs express a unique variant of the FcεRI high-affinity IgE receptor. Unlike mast cells and basophils, which express prodigious amounts of FcεRI on their surface, pDCs express approximately 10-fold less, and the receptor itself lacks a beta subunit.6-8 It is not entirely clear what the specific function of the IgE receptor may be on pDCs, but its expression is thought to contribute to the severity of atopic disease.9-12 It has been proposed that FcεRI-expressing pDCs use IgE for antigen sampling and/or antigen focusing and modulation.13 It is known that pDCs can prime Th1-like responses with secretion of IFN-α and IFN-β.14 The authors have previously shown that TLR9 expression and activation enhance these processes, while IgE cross-linking inhibits these important innate and adaptive immune responses.1,15 Others later reported similar findings by evaluating the function of TLR7 on pDCs, thus indicating that critical virus-induced IFN-α responses of pDCs can be deficient in patients with allergic asthma.16,17
Current literature is mixed on the specific role of pDC-derived IgE signals.18 pDCs express a unique trimeric isoform of FcεRI, a key element of the IgE network comprised of an IgE-binding α chain and a dimer of the common Fc receptor γ chain. It does not express the β chain, which mast cells and basophils have in the tetrameric isoform, responsible for cell signal amplification.16 Increased expression of FcεRI on pDCs correlates with decreased severity of food allergy and asthma, suggesting a role of IgE/FcεRI signals in modulating atopic disease. Furthermore, since TLR9 activation of pDCs attenuates IgE/FcεRI-mediated production of pro-inflammatory cytokines, it suggests a possible role of the pDC in restraining allergic inflammation.16,17 In contrast, allergen immunotherapy increases pDC TLR9-mediated innate immune function, which is impaired at baseline in allergic subjects.15
Given the possible role of pDC IgE/FcεRI in allergic inflammation, the authors aimed to further uncover how IgE may be organised on this cell type. The authors’ preliminary observations suggested that, despite the fact that pDCs display less IgE on their surface, use of the anti-IgE monoclonal antibody, omalizumab, only removed a small portion of IgE from the cell surface. This prompted a new hypothesis that pDC may secure a significant amount of IgE bound within the cell membrane. The presence of membrane-bound IgE (mIgE) on pDCs is not currently reported or known. To achieve this, the authors sought to quantify the presence of membrane-IgE on pDCs by using quilizumab, which is specific for membrane-bound but not receptor-bound IgE.
METHODS
Subject Selection
Blood was drawn from adult volunteers between 2022–2023 at the Johns Hopkins Allergy & Asthma Center, Baltimore, Maryland, USA, according to a protocol approved by the Johns Hopkins Institutional Review Board (IRB). In this cross-sectional study, subjects were selected based on a range of representative serum IgE levels. All subjects were followed in the allergy clinic for some underlying disease such as atopic dermatitis, allergic rhinitis, or asthma. Exclusion criteria included active subcutaneous allergen immunotherapy, immune modulators, oral prednisone, or biologic therapy, such as omalizumab or dupilumab. Other medication status was not known or excluded.
Cell Preparation
Blood specimens were processed as described previously.19,20 Briefly, whole blood in 10 mM ethylenediaminetetraacetic acid underwent centrifugation at 500 g to isolate a buffy coat. Buffy coats were diluted 1:1 (vol/vol) with PIPES albumin glucose-ethylenediaminetetraacetic acid and then subjected to single-Percoll (1.081 g/mL) density centrifugation, producing a peripheral blood mononuclear cells (PBMC) suspension. Platelets were removed from the PBMC suspension using four low-speed (100 g) centrifugations and washes. Cells were fixed in a 1:1 dilution of 2% paraformaldehyde and stored overnight for staining and flow cytometry. To purify basophils and pDCs, a double Percoll (Sigma-Aldrich, St. Louis, Missouri, USA) gradient was used to generate a basophil-depleted cell fraction and a basophil-enriched cell fraction. Basophils were purified to >99% using negative selection antibodies and beads.21 The pDC were prepared from the basophil-depleted cell suspension using blood dendritic cell antigen (BDCA)-4+ selection after conducting two cycles of CD14+ selection to first remove monocytes.22
IgE Competitive Binding of Purified pDCs and Basophils
The authors first more rigorously attempted to remove IgE bound to FcεRI on the surface of pDC with omalizumab in a dose-response fashion to compare this outcome with methods previously utilised for basophils and mast cells.6,23 Omalizumab was used to strip IgE from pDCs and basophils (as a control). Omalizumab concentrations used were 0, 100, 500, and 1,500 µg/mL, which also included omalizumab stock dilution reagents as a control. The authors then used a ‘tube-within-a tube’ culture method by inserting a 1.5 mL tube within a 15 mL culture tube with a loosely fit top so that gas exchange is possible. Basophils were cultured at a concentration of 5×105 and pDCs at 2.5×105 in Complete Iscove’s Modified Dulbecco’s Medium culture medium that contained IL-3 (10 ng/mL). Cells were cultured overnight for 16 hours in a humidified incubator at 37 °C and 5% CO2. The next day, the cells were pelleted, washed three times in phosphate buffered saline, and then checked for IgE expression using flow cytometry.
Antibody Selection
The authors used quilizumab (a mouse anti-human monoclonal IgG1 antibody) to detect the presence of IgE on the membrane of circulating pDCs. Quilizumab is a humanised monoclonal IgG1 antibody directed against the M1 segment exclusively expressed only on human membrane-bound but not receptor-bound IgE. They utilised CD19 and CD27 fluorescent antibodies to focus on a juvenile IgE-switched B cell subset in order to increase the probability of identifying membrane-bound IgE on these cells in the circulation. If mIgE is indeed present on pDCs this may help explain why omalizumab only weakly removes IgE from these cells and may suggest an additional role for pDCs in allergic response.
Flow Cytometry
Approximately 6 mL of blood was drawn from each of six consenting individuals, some of whom have self-reported sensitivity to aeroallergens. PBMCs were isolated using standard methods that have been previously described.19,20 All cells were first prepared for flow cytometry staining by incubating with a non-specific human IgG blocking antibody (1 mg/mL). The cells were split into six tubes: two for B cells and four for pDCs. Target staining antibodies included quilizumab (mouse anti-human IgE, used at 10 µg/mL and obtained courtesy of Genentech, Inc. [South San Francisco, California, USA]), anti-human FcεRI (0.5 µg/mL, AER-37, eBioscience/Thermo Fisher, Waltham, MA, USA), and appropriate isotype controls. B cells were gated for using 1:20 dilution commercial mouse anti-human CD19-Alexa488 (BioLegend, San Diego, California, USA) and 1:20 dilution mouse anti-human CD27-PE (stock 50 µg/mL, BioLegend, San Diego, California, USA), which has been reported as a marker for juvenile B cells. pDCs were gated for flow cytometry with 1:20 dilution commercial mouse anti-human BDCA-2-Alexa488 (stock 200 µg/mL, BioLegend, San Diego, California, USA) and 1:20 dilution mouse anti-human CD123 (IL-3)-PE (stock 200 µg/mL, BD Biosciences, Franklin Lakes, New Jersey, USA). A 1:1,000 dilution of anti-mouse IgG Alexa-647 (stock 1 mg/mL, SouthernBiotech, Birmingham, Alabama, USA) was added for secondary labelling of the authors’ target antibody. The samples were analysed to approximately 600,000 total cells via a BD Accuri C6 Plus flow cytometer, and mean fluorescence intensity (MFI) was measured.
Statistical Methods
A paired t-test was used to compare proportions of counted cells of both the isotype controls and target antibodies. Data are presented as means and confidence intervals, as appropriate. Statistical analysis was performed with InStat software (GraphPad, San Diego, California, USA) by a paired t-test and two-tailed non-parametric Mann–Whitney test. Linear regression and Spearman’s rank formula were used to calculate correlations. Chi-squared test was used to compare counts among counted groups. A P value of <0.05 was considered significant.
RESULTS
Blood obtained from the six adult subjects had total serum IgE levels ranging from 37–1,301 kU/L. As shown in Figure 1A, as a control experiment, omalizumab behaved in a dose-response fashion, with a concentration of 1,500 µg/mL effectively outcompeting all IgE from the surface of basophils. In contrast, while pDCs generally have approximately 10-fold less IgE bound to the surface compared to basophils, omalizumab resulted in incomplete competitive inhibition of IgE (Figure 1B). This suggested that there might be some mIgE on the pDC that wouldn’t be inhibited by omalizumab’s facilitated dissociation characteristic when used at high concentrations. Figure 1C shows the fraction of IgE levels outcompeted from basophils is significantly higher than that of pDCs, which reinforces the finding that IgE is significantly more difficult to out-compete from pDCs than basophils using omalizumab. It should be noted that omalizumab has a relatively low binding affinity for IgE, so it is unlikely that all of these antibodies would be competitively unbound from the cell membranes.
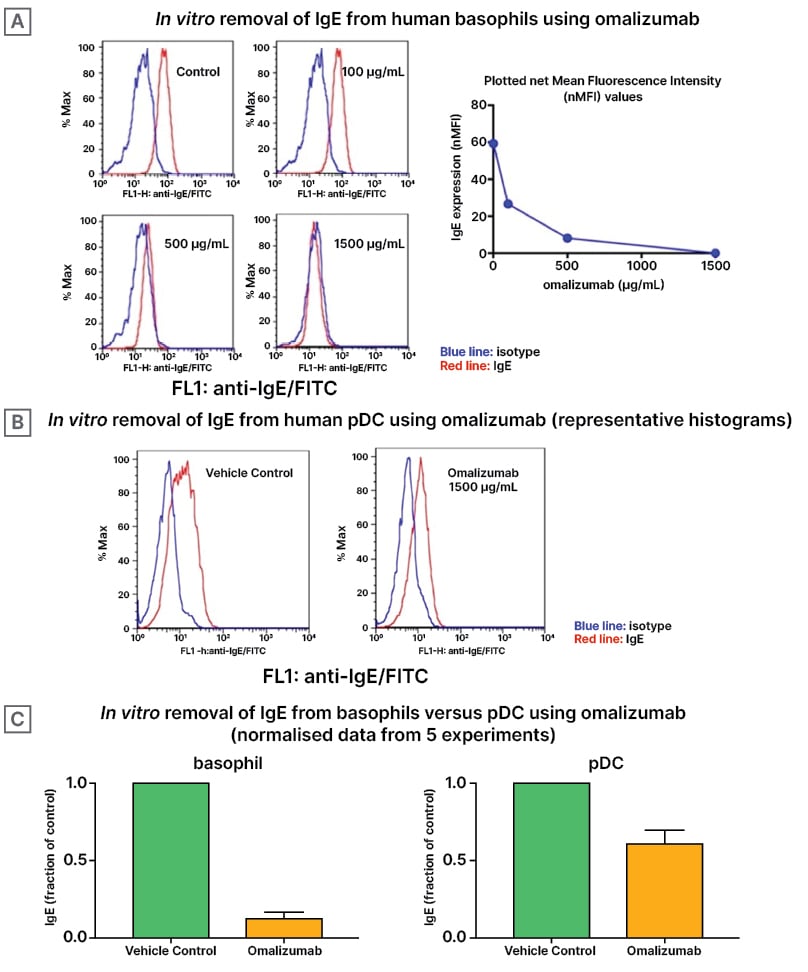
Figure 1: Effects of varying doses of omalizumab on IgE expression.
A) Representative histograms of basophils treated with increasing doses of omalizumab are shown. At 500 and
1,500 mg/mL, nearly all of the IgE was outcompeted (P<0.0001). B) Similar histograms of pDCs treated using a
single maximum high dose of 1,500 mg/mL omalizumab are shown. Even at 1,500 mg/mL, a large proportion of
IgE remained bound to the pDC but did reach significance (P=0.046). C) Comparing basophils and pDCs,
omalizumab outcompeted significantly more IgE from the basophil compared with the pDC (P=0.02).
FITC: fluorescein isothiocyanate; pDC: plasmacytoid dendritic cells.
The primary outcome was to determine the presence of mIgE on pDCs. As shown in Table 1, BDCA-2+ CD123 (IL-3)+ pDCs did not significantly stain positive for quilizumab compared to isotype in the overall proportion or MFI (0.18% versus 0.19%, P=0.125; and 2,448 versus 2,534, P=0.165, respectively). These data indicate that these rare pDCs do not express an appreciable level of membrane-bound IgE in the bloodstream. A representative subject demonstrating the pDC and B cell gating protocols along with quilizumab staining cell selection strategy is shown in Figure 2A-D. The primary outcome was to determine the presence of membrane-bound IgE on pDCs. pDCs are uncommon cells in the peripheral bloodstream, with typically only about 700 pDC per 500,000 total cells in circulation. The number of cells identified as pDC in the authors’ study were likewise rare, amounting to only about 0.18% of total PBMC.
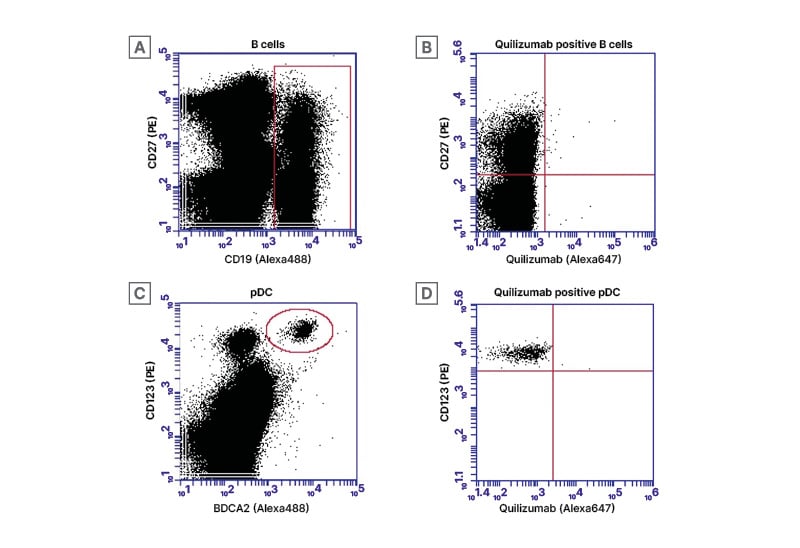
Figure 2: B cell and plasmacytoid dendritic cells gating strategies.
All cells of interest were initially filtered using FSC and SSC gating based on previously published location of B cells and pDCs among PBMCs. A) B cells were then identified with CD19 (Alexa488) and CD27 (PE) indicated by a red rectangular gate. (B) CD19+ B cells were then sub-selected for CD27 (PE) and quilizumab in an effort to identify possible membrane-bound IgE-bearing B cells (upper right quadrant). In a similar manner C) pDC were first identified with BDCA2 (Alexa488) and CD123 (PE) indicated by a red circle. D) Finally, BDCA+ pDC were then sub-selected for CD123+ (PE) and quilizumab to identify possible membrane-bound IgE bearing pDC (upper right quadrant).
FSC: forward scatter; PBMC: peripheral blood mononuclear cells; pDC: plasmacytoid dendritic cells; SSC: side scatter.
As a control experiment, B cells were separated by cell surface markers to determine whether the membrane-bound IgE target antibody was present in higher proportions in certain subtypes of B cells (Table 1). As expected, the proportion of cells staining positive for quilizumab were highest among the CD19+CD27+ B cells compared to CD19+CD27- B cells and CD19-sub-groups (0.17% versus 0.03% and 0.004%, respectively; P=0.008). However, the proportion of cells staining positive for the equivalent isotype-control-stained CD19+CD27+ B cell sub-group was also highest compared to other subtypes (0.12% versus 04% and 0.006%, respectively; P=0.014). Overall, among all CD19+ B cells, there was no significant difference in cells staining positive for quilizumab compared to isotype (P=0.487).
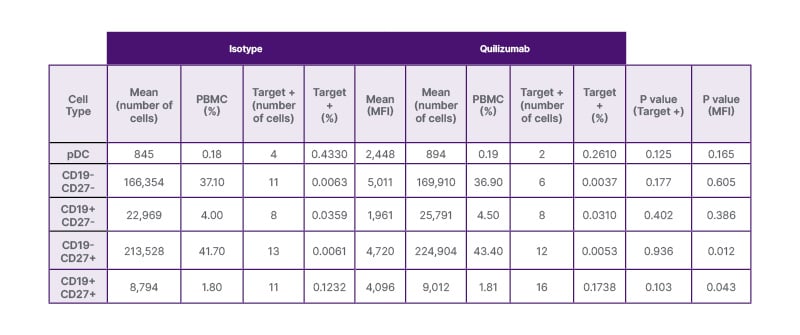
Table 1: Quantification of plasmacytoid dendritic cells and B cells that bind quilizumab (n=6).
There was no difference in the number of BDCA2+ IL3+ pDCs that bound quilizumab compared to isotype (P=0.125). The MFI was also similar between the isotype and quilizumab-bound cells (P=0.165). In a similar manner, CD19+CD27+ and CD19+CD27- B cells, thought to express membrane-bound IgE, did not statistically bind quilizumab more than isotype control (P=0.103 and P=0.402, respectively). Of note, CD19+CD27+ B cells bound quilizumab to a much higher degree than CD19- PBMC at a ratio of 54:1 (P=0.015); however, this was also true, to a lesser degree, for the isotype control with a CD19+CD27+/CD19- PBMC ratio of 32:1 (P<0.001). The rarity of the events may make it difficult to distinguish true positive CD19+CD27+ quilizumab binding from non-specific binding of the isotype, and therefore the possibility cannot be excluded.
+: positive; MFI: mean florescence intensity; PBMC: peripheral blood mononuclear cells; pDC: plasmacytoid dendritic cells.
B cells identified as CD19+CD27+ were targeted specifically in the authors’ study because these cells are thought to more likely express mIgE. Although the total number of CD19+CD27+ B cells for each patient was greater than the number of pDCs, these CD19+CD27+ cells are likewise rare. As shown in Table 1, while there was a trend of higher proportion of CD19+CD27+ cells staining for quilizumab compared to isotype, this did not quite reach statistical significance (0.17% versus 0.12%, P=0.103). The MFI for isotype versus quilizumab were also similar among the CD19+CD27+ B cells, with the isotype actually having a slightly higher MFI (P=0.043).
Given the significantly higher affinity of Alexa647 stain to CD19+CD27+ sub-group cells in both the isotype and quilizumab target groups, and given the expectation that mIgE expressing B cells would be rare, a comparison of those cells to the rest of the PBMC cells (CD19-27-, CD19-27+) was calculated. In both the isotype and quilizumab groups, there was a statistically significant higher proportion of Alexa647 target-bound cells (P<0.001 and P=0.015, respectively), suggesting that these cells appear to have a higher binding affinity than the CD19- PBMC. A ratio of the proportions was calculated for both groups, comparing CD19+27+ to the CD19- cells. Nearly twice as many CD19+CD27+ cells stained positive for quilizumab compared to isotype 54:1 versus 32:1, respectively (P=0.234), although this was not statistically significant due to the small sample size and large variance. There was no significant difference in the proportions or ratio of target cell staining when comparing CD19+27+ versus CD19-27- cells.
DISCUSSION
This study is the first to examine the presence of membrane-bound IgE in both circulating B cells and plasmacytoid dendritic cells using quilizumab, a monoclonal antibody that specifically targets the M1 segment that is exclusive to membrane-bound IgE. Overall, the authors did not identify a significant number of circulating plasmacytoid dendritic cells that bind quilizumab. This study demonstrates that circulating pDCs do not express appreciable amounts of membrane-bound IgE, regardless of the patient’s total IgE level up to 1,300 kU/L. Therefore, the presence, or lack thereof, of membrane-bound IgE on pDCs does not explain their finding that omalizumab insufficiently removes IgE from the surface of pDCs compared to basophils. It is also possible that the antibody could not effectively bind quilizumab due to innate isosteric properties of the antibody interaction.
pDCs are involved in the pathogenesis of multiple allergic conditions, including atopic dermatitis, asthma, and chronic rhinitis, in addition to their antiviral immunity and modulation of the innate immune axis through the production of Type 1 interferons. In the lungs of patients with allergic asthma, for example, pDCs mediate tolerance to inhaled antigen through induction of regulatory T cells compared to conventional DCs that promote Th2 sensitisation to inhaled antigen after reaching the lymph nodes.24 Most studied in allergic asthma, pDCs negatively regulate allergic airway inflammation and Type 2 immune responses via IFN-α and in pDC-deficient mouse models, more severe allergic airway responses developed upon allergen exposure.25 pDCs have also been implicated in atopic dermatitis. Patients with atopic dermatitis had an increased intralesional dendritic cell proportion, with pDCs as the predominant cell type.26 pDC infiltration in these lesions were in close association with blood vessels expressing peripheral neural addressins, thereby altering Th1/Th2 differentiation and leading to more severe disease.
It has been shown, quite interestingly, that the IgE-specific receptor (FcεRI) and TLR9 molecules oppose each other on pDCs.1 pDCs have TLR9 molecules through which ligation with CpG DNA favours Th1 responses. Additionally, while pDCs stimulate allergen-dependent T cell proliferation and Th2 production, CpG-activated pDCs inhibited allergen-dependent proliferation in Th2 memory cells and markedly increased IFN-γ production.27 The results of this study demonstrated that pDCs are not only extremely rare cells in the peripheral blood, but likely do not express membrane-bound IgE. Given the role of pDCs as balance mediators in the Th2-mediated allergic response, a probable postulate is that they do so only through the high affinity IgE surface receptor, FcεRI, and not a membrane-bound form.
The lack of significant binding of quilizumab to pDC does not help explain why IgE is more difficult to outcompete from these circulating dendritic cells compared to basophils. While it has certainly been demonstrated that the FcεRI on pDCs are present in much lower abundance compared to conventional DCs, mast cells, and basophils (with a ratio of about 10:1), the receptor is unique in that it lacks the β-subunit. Omalizumab does not bind IgE already attached to FcεRI on cells;28 therefore, omalizumab would presumably bind circulating IgE in the same manner and with the same affinity whether or not it dissociated from a basophil or a pDC. Jardetzky and his collaborators have shown that omalizumab has the capability of inducing facilitated dissociation.29 The underlying concept is that omalizumab first weakly binds to some portion of IgE not specifically engaged in the receptor pocket, and then a conformation change is induced in the IgE that weakens its association with the receptor, leading to more dissociation. When this occurs, it effectively captures the IgE to prevent re-association, although the concentrations required to induce this behaviour are extreme, i.e., 100 to 1,000-fold above stoichiometric levels. This process may be dependent on the precise nature of the molecular environment of bound IgE on pDCs.
One hypothesis, therefore, is that the unique αγ2 trimeric form of the FcεRI receptor on plasmacytoid dendritic cells binds IgE more tightly, or differently, compared to the αβγ2 tetrameric form expressed on basophils and mast cells. No studies have been done specifically examining the kinetics of these two receptor types. However, supporting the concept that the trimer form of FcεRI is handled differently by the pDCs, several studies suggest that glycosylated FcεRI traffics to the cell surface faster in pDCs compared to basophils30,31 and could sequester IgE more readily. Further, studies in transgenic mice expressing human FcεRI suggest that DCs and monocytes internalise IgE to a greater extent than basophils and mast cells and that overall, IgE is bound less frequently to DCs on the surface.30 How these differences affect the binding and dissociation of IgE on these cells is unknown. More detailed kinetic studies of the interaction of IgE with FcεRI on pDCs and its implications on omalizumab binding need further exploration.
To have a positive control for quilizumab, the authors attempted to use circulating B cells, specifically the CD19+CD27+ subset. However, membrane-bound IgE was determined to be scarce among this cell population. Of note, the CD19+CD27+ cells, which are thought to be a maturing B cell subtype, did, in fact, bind to quilizumab in a much higher proportion compared to the CD19- PBMC (P=0.015) and CD27- B cells (P=0.091). However, while CD19+CD27+ B cells were more likely to bind quilizumab compared to all CD19- PBMC with a proportion of about 50:1, these cells also bound to the isotype control with a proportion of about 30:1, and therefore did not reach significance. Unfortunately, this study may not have been powered high enough to detect a difference in these two assays groups because circulating B cells that possess membrane-bound IgE are presumably exceedingly rare.
One of the strengths of this study is that it presented a novel protocol using an antibody specific to membrane-bound IgE to stain pDCs. Quilizumab has never been used to determine whether pDCs express membrane-bound IgE. One of the potential limitations of the study is the preferential binding of isotype control antibody to CD19+CD27+ B cells causing high background. From the data, the MFI range of the isotype is close to that of the target antibody. It is possible that there may have been a small number of quilizumab staining B cells that were masked by the high background binding of the isotype control. A larger powered study analysing >1 million cells in additional subjects may have identified more target-bound cells or PBMC. Interestingly, like quilizumab, the isotype-control antibody seemed to bind CD19+CD27+ B cells more specifically than other subtypes as well. The selection of another alternative isotype may also have circumvented this.
Overall, this study demonstrates that while circulating pDCs express high levels of FcεRI that very tightly bind IgE on the surface, there is not an appreciable amount of membrane-bound IgE detected. Additional studies will need to further address the specific tight interaction of IgE with the unique high-affinity FcεRI receptor on plasmacytoid dendritic cells.





