Abstract
Introduction: Eosinophilic oesophagitis (EoE) is an emerging disease of the oesophagus. However, so far there are no fully validated biomarkers for diagnosis and monitoring. Moreover, research focuses on parameters that are not very useful and accessible for routine clinical practice. Thus, endoscopy remains the main method of follow-up in this population.
Methods: The team analysed the levels of total Ig E, absolute eosinophil count (AEC), eosinophil cationic protein, and immunoglobulin G4 in a cohort of 399 adult patients with EoE (without other oesophageal pathologies). After controlling for confounding factors, they compared patients with active EoE and those in remission (responders).
Results: It was observed that, in responders, the AEC was lower (p=0.014). Furthermore, in corticosteroid-controlled patients, total Ig E was lower (p=0.016); while in proton pump inhibitors, responders both absolute (p=0.007) and percentage (p=0.002) eosinophils were lower.
Conclusion: The team considers that AEC is probably the most accessible and useful marker for monitoring treatment response in EoE.
Key Points
1. Effective differentiation between active disease and remission in eosinophilic oesophagitis (EoE) is pivotal for guiding clinical management and enhancing outcomes for affected patients.2. The study described is a prospective cohort study. In this type of study, the progression of variables (such as total IgE levels, eosinophil count, eosinophil cationic protein, and immuniglobin G4) is compared between patients with active EoE and those in remission over time to gain a better understanding of the disease and its clinical manifestations.
3. Total Ig E levels, eosinophil count, eosinophil cationic protein, and immuniglobin G4 are promising markers to distinguish between active and remission phases of EoE, offering potential for improved clinical monitoring and treatment management.
INTRODUCTION
Eosinophilic oesophagitis (EoE) is an immune-mediated condition affecting the oesophagus, clinically characterised by symptoms of oesophageal dysfunction and histologically marked by predominant eosinophilic inflammation.1 It has been reported to have a prevalence of 34.2 cases per 100,000 person-years, and an overall incidence rate of 4.4 new cases per 100,000 person-years.2 Onset can occur at any age, with a peak in adults aged 30–50 years.1,2 It is currently the most common cause of food bolus impaction in the oesophagus in patients attending the emergency department.1 Although conditions such as rhinitis, asthma, and eczema are more prevalent in this population, atopy has not been demonstrated as a predisposing factor.1,2 Various clinical presentations (phenotypes) and underlying pathogenic mechanisms (endotypes) have been recently described.3 Proton pump inhibitors (PPI), topical corticosteroids (TC), and an empirical diet (ED) can be offered as first-line anti-inflammatory treatments.1 Upper gastrointestinal (GI) endoscopy remains the gold standard for diagnosis and monitoring, involving multiple biopsies that reveal a density of ≥15 eosinophils per high-power field under light microscopy.1-3 Hence, the quest for new non-invasive biomarkers is a significant objective. In this study, the team compared variations in total IgE levels, absolute eosinophil count (AEC), eosinophil cationic protein (ECP), and immunoglobulin G4 (IgG4) values between patients with uncontrolled (active) EoE and those with controlled disease (remission).
MATERIALS AND METHODS
The team conducted a prospective analysis of patients aged 18 years or older who were diagnosed with EoE and treated at the allergy department of Hospital Universitario La Paz in Madrid, Spain. Patients were selected after excluding any concurrent oesophageal pathologies (as confirmed by the gastroenterologist unit). This study received approval from the hospital’s ethics committee under protocol number PI-5401.
Patients with a documented history of atopic comorbidities in remission or under control and who did not require systemic treatments, such as corticosteroids or biologics, were specifically included. Patients with concurrent medical conditions or undergoing treatments that could potentially influence the serum parameters under investigation were excluded from the study.
For the purpose of analysis, the patients were categorised into three groups based on their prescribed treatment regimens: PPIs Group, patients receiving omeprazole at a dosage of 20 mg every 12 hours; TC Group, patients administered fluticasone (Flixonase®) at a dosage of 400 µg every 12 or 24 hours; and ED Group, patients managed with an empirical diet comprising either three or six food groups, in accordance with the team’s clinical practice protocol. These food groups included milk, wheat, and egg, as well as legumes, nuts, and fish/seafood.
They assessed treatment response by performing upper GI endoscopy and collecting multiple biopsies. For patients in the PPIs group, response evaluation occurred at 8 weeks; whereas for those in the TC and ED groups, assessment was conducted at 12 weeks following the initiation of treatment.
All data processing procedures were closely monitored and overseen by our biostatistics department. In the descriptive analysis, we presented the variables as medians and interquartile ranges. They employed the Mann-Whitney test to compare variables, and statistical significance was established at a threshold of p<0.05.
RESULTS
The study included 399 patients. Table 1 consists of the description of the population’s characteristics. The study population was relatively young, with a median age of 33 years (interquartile range 23–42). A majority of the patients were male (74% [n=296]), and 76% of them (n=304/399) had a history of atopy. Furthermore, the majority of patients (78% [n=272/351]) responded positively to one of the selected treatments. In Table 2, the team conducted a comparison of patients concerning remission histological criteria. It was observed that patients in remission (defined as having <15 eosinophils per high-power field) were generally older at the time of the study (p=0.019) and exhibited lower AEC values (p=0.014). Finally, Table 3 presents the variation in serum parameters based on the treatment received.
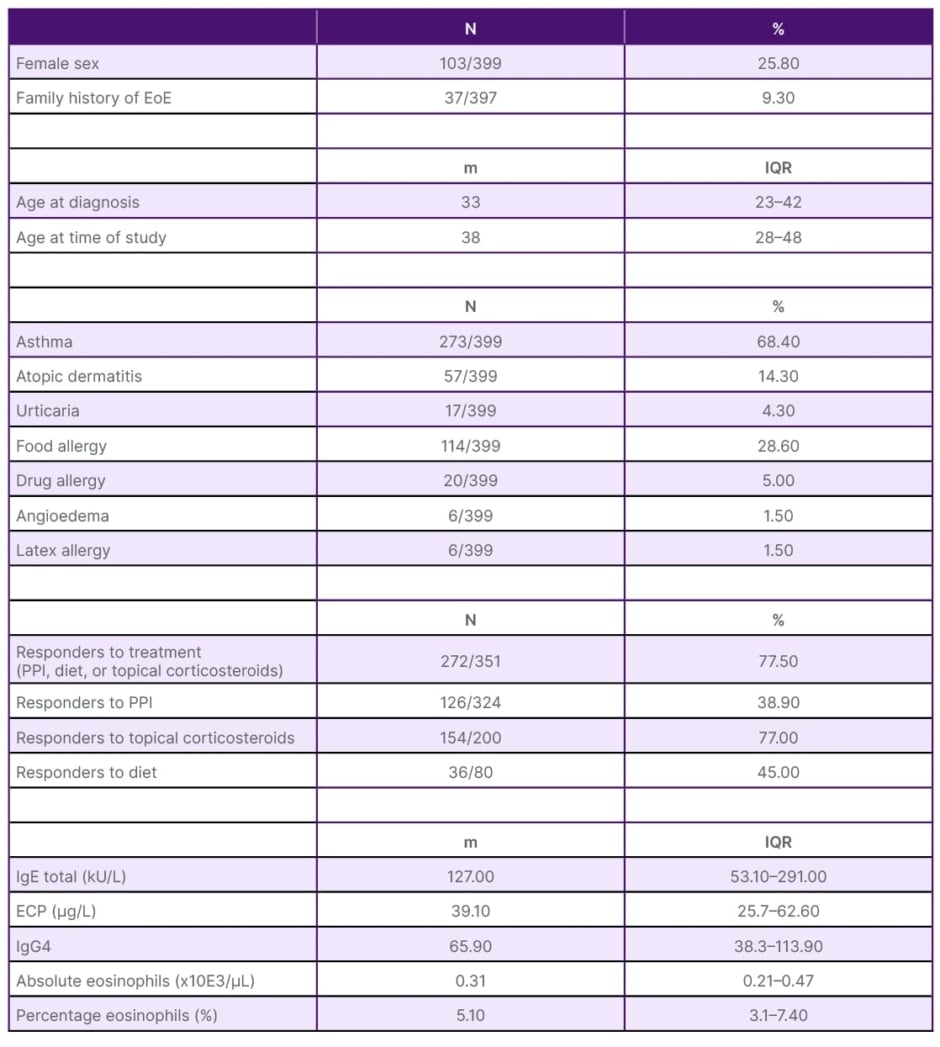
Table 1: General characteristics of the population.
ECP: eosinophil cationic protein; EoE: eosinophilic oesophagitis; IgG4: immunoglobin G4; IQR: interquartile range; m: median; N: number; PPI: proton pump inhibitors.
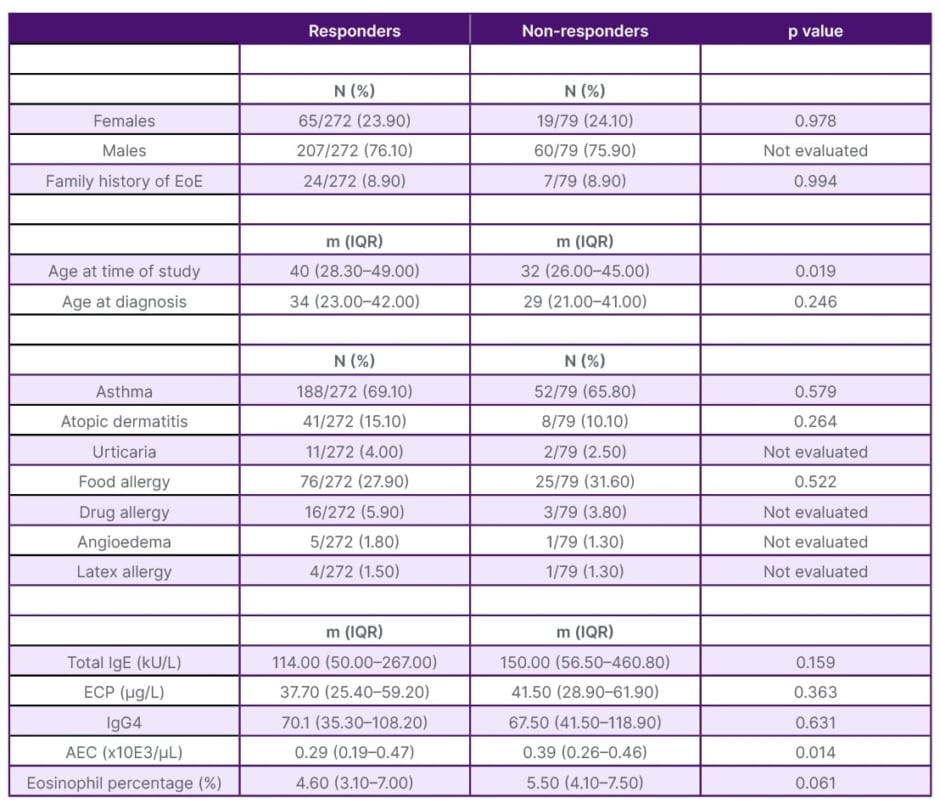
Table 2: Comparison between responders and non-responders.
AEC: absolute eosinophil count; ECP: eosinophil cationic protein; EoE: eosinophilic oesophagitis; IgG4: immunoglobin G4; IQR: interquartile range; m: median; N: number.
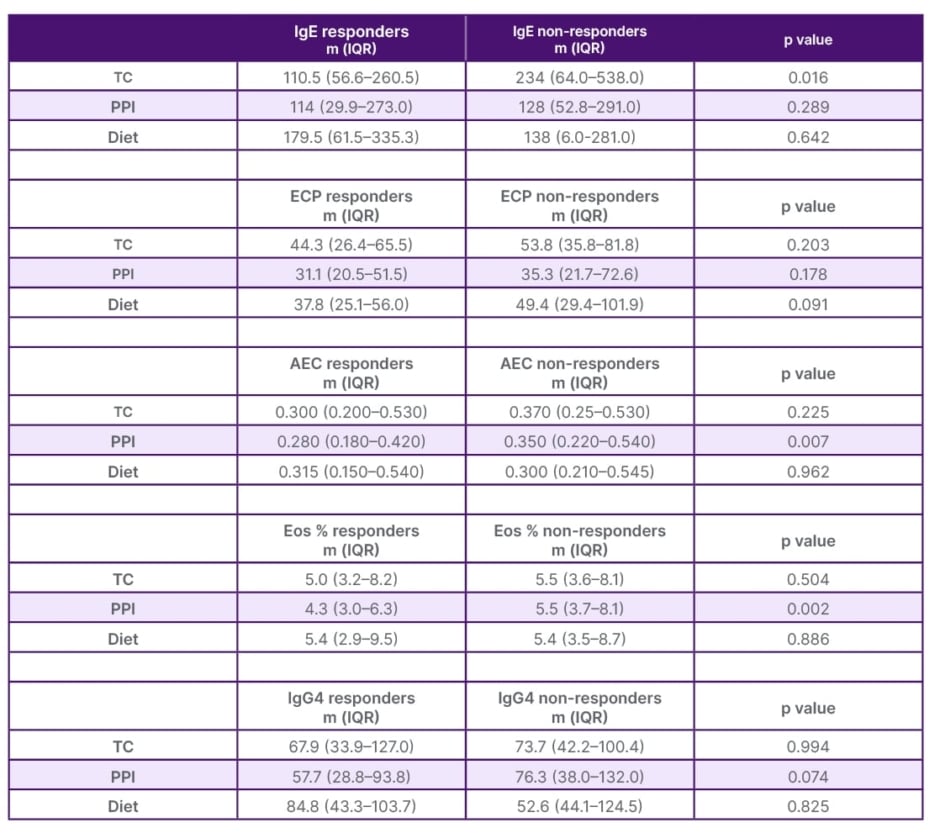
Table 3: Comparison of markers by treatment group.
AEC: absolute eosinophil count; ECP: eosinophil cationic protein; Eos: eosinophils; IgG4: immunoglobin G4; IQR: interquartile range; m: median; PPI: proton pump inhibitors; TC: topical corticosteroid.
DISCUSSION
EoE is a chronic inflammatory disease whose diagnosis and follow-up currently require multiple oesophageal endoscopies.4 The identification of non-invasive, accurate, and reliable biomarkers remains a challenge.5 In this regard, Dellon et al.6 analysed a large panel of serum biomarkers but did not identify useful markers for the treatment of the disease. Reporting of new data on the pathophysiology of the disease has led to the identification of promising markers, although few can differentiate EoE from other atopic diseases.7 In addition, significant oesophageal infiltration of Ig G plasma cells and elevated levels of IgG4 have been identified in these patients, although their contribution to EoE is unclear.8,9 No robust and definitive biomarker for the management of EoE has yet been identified, although many of the data point to AEC and, more recently, ECP.4,5
General data for this study’s population (Table 1) coincide with the characteristics already described in these patients, such as association with multiple atopic comorbidities.4 Table 2 shows that in the responder group, patients were older at the time of the study (p=0.019); older age could improve adherence to treatment. AEC was also lower in responders (median 290 eos.×10E3/µL; p=0.014). While the percentage of eosinophils showed a non-significant trend (p=0.06). That difference is probably due to the low AEC. Perhaps in larger studies, this difference will be clearly significant. Thus, the team could propose an AEC value (both absolute and percentage) at which EoE could be considered controlled or uncontrolled. In this regard, Rodríguez-Sánchez et al.10 reported a significant decrease in eosinophils although they assessed patients treated with diet alone.
Schlag et al.11 reported a decrease in AEC and ECP after treatment with TC. Furthermore, Min et al.12 demonstrated an association between AEC and ECP in the diagnosis of EoE, although only AEC could predict oesophageal eosinophilia after TC treatment. Subsequently, Wechsler et al.13 reported that the combination of AEC, ECP, and other serum parameters was superior in the differential diagnosis of EoE. Doménech Witek et al.14 reported that serial determination of ECP would be useful in the follow-up of EoE, while Cengiz15 reported on the potential of ECP in the diagnosis of EoE. Despite the promising results of ECP,11,14 the team found no differences, probably due to the strict control of potential confounders during patient selection.
When analysing the markers with respect to their response to treatment (Table 3), it was observed that total IgE was significantly lower in patients responding to TC (p=0.016). This finding is interesting considering that the selected population had atopic comorbidities in remission or were fully controlled. This variable has been rarely investigated because IgE does not seem to be involved in the pathophysiology of EoE. Rodríguez-Sánchez et al.10 did not detect changes in total IgE or ECP, probably because their cohort was only treated with diet.
The team observed that AEC and percentage were significantly lower in patients who responded to PPIs (p=0.007 and p=0.002, respectively). Few articles report similar findings, given that most patients received empirical PPIs prior to the diagnosis of EoE. They believe that this type of drug may favour a noninflammatory environment, thus potentially explaining these findings.
Weidlich et al.8 observed higher levels of IgG4 in patients with EoE, although these decreased after treatment with TC. Rizvi et al.9 on the other hand, reported more frequent positivity of IgG cells among paediatric patients who responded to PPIs. This study reported no significant differences.
CONCLUSION
Consequently, despite the limitations in methodology, the data imply that AEC, particularly in patients treated with PPIs, represents a promising and readily accessible marker for monitoring treatment response in individuals with EoE. Nevertheless, additional studies are required to validate our findings.





