Abstract
Allergen-specific immunotherapy (AIT) works well both in children and adults. An often-alleged gap between the level of evidence of AIT efficacy in adults versus children is based upon the flawed ‘children-are-not-small-adults’ and ‘children-are-therapeutic-orphans’ mantras, both of which emerged in the 1960s. These mantras led to paediatric legislation in the USA in 1997 and the European Union (EU) paediatric regulation 10 years later. Although preterm newborns and newborns are vulnerable, during the first year of life their organs mature. Young children are no longer physiologically newborns; their immune system can overreact and cause allergic reactions, and AIT works for them just like it does in adults. Young patients need dosing recommendations and safety observations, rather than repetition of proof of efficacy. Placebo-controlled efficacy studies withhold effective treatment, increase the risk of asthma in the placebo group and are, therefore, in the authors’ opinion, unethical as well as in breach of the declaration of Helsinki. Individuals under the age of 18 years are not offered AIT treatments that are available to adults that are 18 years or older, but AIT treatment would be a suitable option. Since 2007, there were >100 EMA paediatric investigation plans that demanded ‘paediatric’ AIT studies involving tens of thousands of minors. Almost none were successfully undertaken and those that were done were unnecessary. It is time for the specialty of allergy to face this challenge.
INTRODUCTION
Allergology has struggled with the issue of whether children and adults are both successfully treated with allergen immunotherapy (AIT).1 Some allergists emphasise the gap between the level of evidence of the efficacy of AIT in adults versus children.2 The European Academy of Allergy and Clinical Immunology (EAACI) Future of the Allergists and Specific Immunotherapy (FASIT) working group published a consensus position in 2018. It criticised the European Medicines Agency (EMA) demands for 5-year double-blind placebo-controlled AIT efficacy trials in children that, in the placebo group, would prevent effective treatment and increase the risk of asthma.3 However, it also attested to the European Union (EU) paediatric regulation that it enshrines the right for children to receive evidence-based medicine.4 The EMA emphasises that children react differently to medicines and that, therefore, medicines need to be properly studied for safety and efficacy in minors.5 Preterm newborns are indeed vulnerable and immature compared with adults. But minors mature physiologically well before their 18th birthday.6,7 The reasons why many paediatric researchers are reluctant to apply the learnings of developmental pharmacology and physiology to the medical treatment of minors is based upon conflicts of interest that have slipped into the triangle of academic research, regulatory authorities, and the life science industry. In several large clinical areas, careers have been built on ‘paediatric’ studies, justified by the ‘children-are-not-small-adults’ and ‘children-are-therapeutic-orphans’; mantras.8-10 Allergology was not the core area where these mantras emerged. Rather, allergology was overrun by the wave of EMA-demanded paediatric studies from 2007.11 Nevertheless, as long as this contradiction remains unaddressed in the world of allergology, it will persist.
HISTORY OF PEDIATRIC LEGISLATION IN THE USA AND EUROPEAN UNION
Labels describe the content of a product. After 1900, drug labels in the USA became more accurate regarding content and medical qualities.12 However, labels are not instructions for physicians. Physicians can prescribe what they think will help their patient. Labels in the USA blocked trading of adulterated food and drugs. From 1962, manufacturers in the USA inserted paediatric warnings into drug labels to prevent damaging lawsuits in the litigious legal environment of the country, emphasising that no paediatric studies had been performed. These warnings were based on toxicities observed in preterm newborns treated in the 1950s with antibiotics, and on the U.S. Food and Drug Administration’s (FDA) increased power with the new USA pharmaceutical legislation of 1962, reacting to the thalidomide catastrophe.12 Since 1962, USA law requires proof of efficacy and safety of drugs before approval. Today, this principle is recognised worldwide.13 Interpreting the paediatric warnings, the first chairman of the American Academy of Pediatrics (AAP) committee on drugs characterised children as “therapeutic orphans.”14 Furthermore, the AAP desired funds for paediatric research.
The term ‘off-label’ emerged in 1988.15 The FDA prosecutes companies that encourage off-label use and discourages discussion of off-label use.12 In 1977, the AAP complained that many drugs were not allowed to be advertised for children. In 1979, the FDA defined ‘children’ as <17-year-old. Since 1997, the USA rewards paediatric studies; since 2003, the FDA can mandate paediatric studies also without rewards.8-10,16 The authors have used inverted commas because adolescents, for which the FDA requests many ‘paediatric’ studies, are physiologically no longer children, and school-age children 5–11 years of age are no longer newborns.
The EU paediatric regulation has been in effect since 2007. Also, EU researchers had desired more paediatric research. The EU regulation is currently more demanding than the regulation in the USA. For new drugs, companies must negotiate ‘paediatric investigation plans’ (PIP) with the EMA, committing to paediatric studies in persons <18-year-old. In contrast to the USA, paediatric studies are also demanded for rare diseases, vaccines, and biologics.
THE HISTORY OF ALLERGEN IMMUNOTHERAPY PRODUCTS
For a long time, AIT products had been classified as individual products that only needed a production licence. However, European attitudes towards AIT had been different. The Nordic countries developed a standardisation of AIT products. In England, UK, AIT almost disappeared after anaphylactic reactions led to patients’ deaths. Germany became the largest European AIT market, and its Paul-Ehrlich-Institut (PEI) had the highest competence among European authorities. The major AIT producers had developed their own systems for standardisation. In 1989, the EU classified AIT products as drugs, subjecting them to drug approval.17,18 Details remained under the authority of individual states and, for a while, things carried on as before. PEI and German clinicians disliked the multitude of products of different quality. Two paths ensued. Switzerland, which is not part of the EU, allowed the retrospective approval of AIT products with the exception of recombinant allergens.19 In contrast, the central EU paediatric regulation and a German initiative resulted in a challenge that, even today, is not resolved.
In 2008, Germany introduced an ordinance requiring new registration of AIT products as drugs (Therapieallergene-Verordnung).20 Without submitting a marketing authorisation application, AIT products lost marketability after 3 years. If an application was submitted, the PEI could extend the deadline for clinical data submission. AIT products were now confronted with the EMA crusade for paediatric studies. The EMA and PEI jointly developed a ‘standard PIP’, now in its fourth version.21 It mandates: a dose finding study in adults; a 1-year dose-finding-study in children or a justification why data can be extrapolated from adults; and for one leading product a double-blind 5-year placebo-controlled efficacy paediatric study (3 years for treatment and 2 years for follow-up) from each manufacturer. The AIT PIPs either demand separate studies in 5–11 and 12–17-year-olds, or a study in 5–17-year-olds with separate cohorts. Fifty-eight such 5-year double-blind placebo-controlled studies were demanded on the basis of the PIPs issued in 2010, respectively.22 Together the 2 x 58 studies would have required tens of thousands of patients.23
The FDA does not mandate separate paediatric trials for AIT products. Four sublingual AIT products are today FDA-approved as drugs: Grastek (timothy grass pollen [ALK-Abelló, Hørsholm, Denmark]) from ≥5 years of age on; Oralair (grass allergens of sweet vernal, orchard, perennial rye, timothy, and Kentucky blue grass [Stallergenes Greer, London, UK) from ≥10 years on; Odactra (house dust mites [ALK-Abelló]); and ragwiteck (short ragweed pollen [ALK-Abelló]) in patients aged 18–65. These approvals are based on the age of the patients in the respective pivotal studies.
Apart from some small AIT studies,24 very few paediatric studies have been performed for AIT in the last 15 years. Table 1 lists the six large completed paediatric studies with sublingual immunotherapy (SLIT). One study in house dust mites in patients aged 5–17 years was terminated following a drug safety monitoring board decision (NCT01199133).36 In addition, an Oralair safety observation study was performed on 307 5–9-year-olds (NCT02295969)37 and a Phase I safety study of house dust mites in 37 12–17-year-old patients (NCT01919554;38 Table 1).
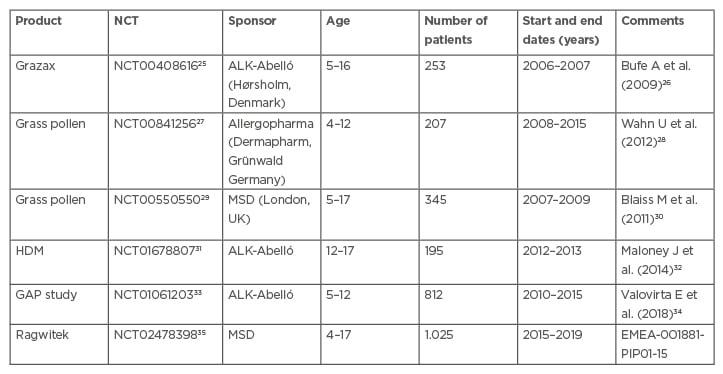
Table 1: Completed sublingual immunotherapy allergen-specific immunotherapy paediatric studies.
The EMA PIP decisions can be retrieved on the internet by entering the PIP number into any search engine.
GAP: grazax asthma prevention; HDM: House dust mites; PIP: paediatric investigation plan.
One paediatric SLIT study is ongoing (NCT03654976).39 It is PIP-demanded (EMEA-001258-PIP01-11-M03) and placebo-controlled. Sponsored by ALK-Abelló, it has recruited 600 patients aged 5–17 years-old with asthma caused by house dust; it is no longer recruiting. Several more studies investigate AIT in both young and adult patients.
An oral immunotherapy product against peanut allergy, Palforzia (Aimmune Therapeutics, Brisbane, California, USA), was investigated in patients aged 4–55 years.30 However, efficacy was shown only in patients aged 4–17.41 Another PIP-demanded study was performed only in minors.42 Today, Palforzia is FDA/EMA-approved in patients aged 4–17 and those becoming 18 during therapy.
Table 2 lists the PEI-approved AIT products.43 Not one single subcutaneous Immunotherapy (SCIT) product received PEI paediatric approval after 2005. Of the 14 tree pollen SLIT 14 products, itulazax is from ALK-Abelló, with seven more itulazax products from various other companies. Two AIT products (SUBLIVAC® Birch and SUBLIVAC Trees (HAL Allergy Group, Leiden, the Netherlands) were approved in 2018. Four staloral products were PEI-approved in 2004 and 2005. Of the 32 grass pollen SLIT products available, 11 are grasax products from various companies; 20 oralair products from various companies, and ragwizax from ALK-Abelló. Of the 10 house dust mice SLIT products, 5 acarizax products are from various companies; Aitaro and Amitend from ALK-Abelló; and 3 Orylmyte products from Stallergenes Geer.
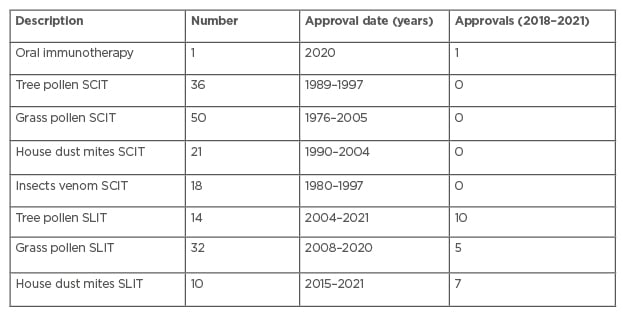
Table 2: Paul-Ehrlich-Institut-approved allergen-specific immunotherapy products in December 2021.
SCIT: subcutaneous Immunotherapy; SLIT: sublingual immunotherapy.
To this day, there is a long list of AIT products that may still be marketed in Germany. They are not approved as drugs, but their marketing is tolerated for now.44 EU-wide there are many more AIT products that still have a national marketing permission.45 The ‘paediatric’ negotiations between the companies that sell these products and the authorities are ongoing. These products are still tolerated because, after the introduction of the Therapieallergene-Verordnung ordinance, the companies applied for an approval as drugs.9-11 There is a discrepancy between the multitude of EMA-demanded ‘paediatric’ AIT studies, the few initiated studies, and the fact that not one single SCIT product has been licensed in the allegedly paediatric population. Only SLIT studies were performed, initiated for products already FDA-approved as drugs.
Not all children are at risk from more toxicities, as claimed in the preamble of the EU paediatric regulation.4 Clinical reviews list for AIT a lower age limit of 5 years, based on pragmatic considerations, and a contraindication for <2 years.46 Allergy reviews also reveal the trouble many authors are experiencing trying to manoeuvre between the official regulatory situation and medical common sense.3 Some authors appear to focus only on technical and clinical questions without addressing the current regulatory conundrum.1
Newborns and preterm infants are vulnerable. Within the first year of life, the organs that are responsible for absorption, distribution, metabolism and excretion mature.7 Neonatal toxicities are no longer threatening. All drugs are dangerous in wrong doses. In the authors’ view, young patients need child-friendly formulations and correct doses, rather than a separate proof of efficacy.8-10
USA-rewarded paediatric studies predominantly examine the use of drugs for adults in minors, rather than the medical needs of children.47 Five years of placebo treatment in an allergy study that denies effective treatment will allow the potential progression to asthma in the placebo group and result in harm to the patients enrolled in the control group. The only reasonable justification for placebo-controlled efficacy trials would be if an adolescent patient’s body and their immune system would undergo a fundamental metamorphosis at their 18th birthday, which would make it necessary to re-assess the efficacy of AIT in adolescents. However, the 18th birthday is only an administrative age limit, which does not correspond to a physiological change of the body. The majority of clinicians feel strongly about using the evidence of clinical studies as opposed to empirical evidence, which was satirised in a paper that questioned the efficacy of parachutes; double-blind randomised placebo-controlled trials are not needed to prove the efficacy of parachutes.48 Critical papers,8 a critical review of PIP-demanded AIT studies,11 and two textbooks have recently been published on this topic.9-10 FDA- and EMA/PIP-demanded paediatric studies are performed worldwide. Many paediatric AIT studies are based upon the uncritical and blurred FDA/EMA classification of children, which contradicts physiology and common sense.6-8 The EMA admits that there are not enough patients worldwide for PIP-demanded studies.49 A paper by FDA authors revealed that 94.5% of all drug adolescent dose recommendations were identical to adults.50 In the authors’ view, adolescents are legally still minor, but already mature physiologically.
In several clinical areas, the FDA has relented from its demand for separate paediatric studies, including in those adolescents who need medications for malignancies and antiepileptic drugs.9-10,51,52 In contradistinction, the EMA has extended its demand for paediatric studies into adult diseases that occasionally occur before the 18th birthday, including amyotrophic lateral sclerosis, hepatic carcinoma, kidney carcinoma, and more.9-10,53
Today, most AIT studies that are not triggered by PIPs recruit both adults and minors. Some large companies decided that the financial investment to perform paediatric studies is worthwhile (Table 1). Participation in such studies results in participation at international investigator meetings, conference presentations, and funding. The results are often published in high-ranking journals. These studies promote paediatric careers in the FDA, EMA, pharmaceutical industry, and clinical research organisations. Allergology was not a clinical area with key clinical representation involved in shaping USA and EU paediatric laws.9-10
The young child at the beginning of the allergy career is prone for plasticity of the immune response, an argument often emphasised in textbooks and articles on AIT, with particular focus on children. This fact is an additional argument against demanding placebo-controlled proof-of-efficacy studies in minor patients.
In 2011, the European Academy of Allergy and Clinical Immunology (EAACI) tried to find a consensus with the EMA,54 but did not address the key challenge: the blur between legal and physiological characterising of children.8-10 After this meeting, EMA employees published a paper that documents how ‘close collaboration’ represents compliance to the association.22 An EMA position paper still claims that off-label use in children is always dangerous,55 without mentioning the life-saving achievements of paediatric oncology, neonatology, and further paediatric sub-disciplines that emerged off-label before the term off-label even existed.15
The EU paediatric regulation enforces additional regulatory studies in children that are not physiologically defined, neither vis-à-vis drug treatment nor vis-à-vis AIT, but chronologically, as <18 years of age. There are indeed fundamental differences between the physiology of preterm newborns and adults. But babies do not remain as immature and vulnerable as preterm newborns until their 18th birthday. The adolescents body is mature well before the 18th birthday, resulting, for example, in identical dose recommendations for adolescents and adults that resulted from many paediatric studies in adolescents, as reported by FDA authors.44 The main changes in children’s bodies and metabolism occur during the first 2 years of life, an age range in which AIT is not carried out.46 The FASIT review repeats flawed EMA assumptions. The medical sense of 5-year double-blind placebo-controlled studies is criticised, as are PIP-demanded AIT paediatric studies in general.3 In the authors’ opinion, the conclusion that stakeholders must further discuss this issue is not sufficient. As long as EMA statements are taken at face value and conflicts of interest are not addressed, this issue will not be resolved.
The FDA has for decades prevented thalidomide-like catastrophes in drug development. Nevertheless, the artificial separation of adults and children has triggered a new worldwide challenge in medical research. Separate AIT paediatric regulatory studies are not only medically unnecessary, but also deny effective therapy to the control group, thereby increasing the risk of asthma. Although the authors have focused on the consequences for AIT, this challenge touches drug development in all clinical areas.8-10
The EMA PIPs have not advanced AIT in young patients.
CONCLUSION
Paediatric allergy should distinguish medically reasonable from unreasonable regulatory studies. Statements that there are limited paediatric data could be considered flawed in the context of this paper. Institutional review boards or ethics committees should suspend these ongoing unnecessary studies. Eventually, USA and EU paediatric laws need to be changed. The representative organs of paediatric allergology should distance themselves from questionable and potentially harmful studies. Allergologists should recommend to their colleagues who consider participation in allegedly paediatric studies to weigh in the benefit and risks for the patients, themselves, and the reputation of paediatric allergology. As everywhere in medicine, doctors in paediatric allergology have to differentiate between scientific findings, the requirements of the regulatory authorities, and the propaganda of drug manufacturers. It is their responsibility both to underage patients and to their parents. The described challenge is not limited to allergology. It is a challenge at the interface between medicine and drug approval in general. But, as the authors have shown above, the demand by the EU regulatory authorities for questionable studies in children in the field of AIT is an extreme example that paediatric allergology has not yet adequately addressed. It is both a challenge and an opportunity for paediatric allergology to demonstrate its vigilance and independence.





