Abstract
One of the complications of systemic juvenile idiopathic arthritis (SJIA) is macrophage activation syndrome (MAS), which may be considered as a form of secondary haemophagocytic lymphohistiocytosis. Trigger factors are drugs (aspirin, nonsteroidal anti-inflammatory drugs, gold preparations, methotrexate, and tumour necrosis factor blocking agents), drug change, drug side effects, or initiation of biological drugs and infections. The pathogenesis of MAS is still unclear and it may be explained by uncontrolled activities of macrophages. A lot of proinflammatory cytokines such as tumour necrosis factor-α, interleukin (IL)-1, IL-6, and interferon gamma play important roles in the pathogenesis of MAS. The diagnosis of MAS is often challenging. In 2016, the European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) approved classification criteria for MAS complicating SJIA. Corticosteroid and cyclosporin A treatment have been used in the treatment of MAS. Intravenous immunoglobulin treatment has been used in some cases of MAS. Biologic agents have been used, such as anakinra (IL-1 alpha and beta inhibition), canakinumab (IL-1 beta inhibition), and tocilizumab (IL-6 inhibition). Early detection and early intervention are vital to avoid poor outcomes in MAS. SJIA is a subtype of juvenile idiopathic arthritis, and MAS is a serious, potentially fatal, complication of SJIA that occurs most commonly in children.
SYSTEMIC JUVENILE IDIOPATHIC ARTHRITIS, MACROPHAGE ACTIVATION SYNDROME, AND HAEMOPHAGOCYTIC LYMPHOHISTIOCYTOSIS
Systemic juvenile idiopathic arthritis (SJIA) is a subtype of juvenile idiopathic arthritis (JIA), which is characterised by arthritis of unknown origin and extra-articular symptoms like spiking fever, often accompanied with a macular rash, serositis, hepatosplenomegaly, and generalised lymphadenopathy due to reticuloendothelial involvement.1 The prevalence rate of the disease varies widely in different series (3.5–5 cases/ 100,000) and the yearly incidence varies from 0.4–0.9 cases per 100,000 children.2 According to the JIA criteria of Edmonton made by the International League of Associations for Rheumatology (ILAR) in 2001, SJIA is defined as arthritis affecting one or more joints, usually in the juvenile age group (<16 years of age), with, or preceded by, fever of ≥2 weeks duration that is documented to be daily (quotidian) for ≥3 days, which may be associated with evanescent (non-fixed) erythematous rash or generalised lymph node enlargement or hepatomegaly, splenomegaly, both, or serositis.2
Macrophage activation syndrome (MAS) is most common in SJIA, and it has been reported that the frequency of marked MAS is ~10%, but it is known that the frequency of MAS, which is not reflected in clinical practice, reaches up to 30–40%.3 It also can occur in patients with other rheumatic disorders such as Kawasaki disease, adult-onset Still’s disease, Behçet’s disease, systemic lupus erythematosus, and juvenile dermatomyositis.2,4,5 Additionally, in the case of an adolescent boy with human leukocyte antigen, B27-positive ankylosing spondylitis has been reported,6 and it is typically characterised by high fever, lymphadenopathy, and hepatosplenomegaly and is variably associated with haemorrhage as well as signs of liver, central nervous system, and kidney involvement, and may lead to multiple organ failure.7,8 This can be characterised by hyperferritinaemia, transaminitis, hypofibrinogenaemia, and hypertriglyceridaemia.
MAS is also a form of secondary haemophagocytic lymphohistiocytosis (HLH) associated with rheumatologic conditions.9 HLH has two forms: primary, or familial HLH, which is autosomal recessive,9 and secondary HLH, which may occur in patients with infections such as Epstein–Barr virus, haematological malignancies, metabolic conditions, and a range of autoimmune and other inflammatory conditions.10 MAS is classified among the secondary forms of HLH. The diagnostic criteria of HLH was based on the HLH 2004 diagnostic guideline. It follows the presence of a molecular diagnosis with specific gene mutations (PRF, UNC13D, STX11) that are associated with HLH or meet five out of eight clinical and laboratory diagnostic criteria including fever, hepatosplenomegaly, cytopenia, hypertriglyceridaemia/hypofibrinogenaemia, haemophagocytosis, low or absent natural killer (NK) cell activity, ferritin >500 ng/mL, and soluble CD25 >2,400.11 The diagnosis requires five out of the eight criteria to be fulfilled, but patients with a molecular diagnosis consistent with HLH do not necessarily need to fulfil the diagnostic criteria.9
Macrophage Activation Syndrome and Genes
Mutations in the perforin (PRF1) gene can be linked to MAS. PRF1 mutations play a role in the development of MAS in SJIA patients. PRF1 is a protein that mediates cytotoxic activity of NK and T cells, and its mutations can lead to NK dysfunction. It is therefore tempting to assume that (heterozygous) mutations in other genes involved in the pathogenesis of HLH, such as genes encoding Syntaxin11 and MUNC13-4, could also be involved in the development of MAS in SJIA patients. Heterozygous protein-altering rare variants in the known genes (LYST, MUNC13-4, and STXBP2) were found in some patients.12,13,14
MACROPHAGE ACTIVATION SYNDROME CAUSE AND PATHOGENESIS
MAS may be triggered as a complication of an underlying disease without any triggering factor or in relation to any infection, or drug side effect.3,15 In MAS, acute infections show that an additional contribution must be provided by the inflammatory status of the patient; indeed, the majority of MAS episodes occur during active disease phases or at disease onset.7 Minoia et al.15 reported that MAS was believed to be related to a treatment side effect (8 of the 11 instances involved a biologic agent).
The pathogenesis of MAS is still unclear. The development of MAS is characterised by a cytokine ‘storm’, with the elaboration of numerous proinflammatory cytokines.16 It has been explained that the over-activated T lymphocytes and macrophages are found in various organs, and perforin deficiency and some cytokines, such as tumour necrosis factor-α (TNF-α), interleukin (IL)-1, IL-6, and interferon gamma (IFN-γ), play roles in the pathogenesis of MAS. Perforin is a cytotoxic protein that lymphocytes secrete to kill virus-infected cells and has the function of controlling lymphocyte proliferation. Therefore, perforin deficiency may lead to persistent lymphocyte activation.2
IL-1β is a proinflammatory cytokine produced primarily by monocytes and macrophages, and it is believed to be central to the pathogenesis of SJIA. IL-1β signals through its receptor and causes lymphocyte and endothelial activation as well as production of other inflammatory cytokines, including IL-6.16
IL-6 is a pleotropic cytokine produced in the early stages of inflammation and is central in driving the acute-phase response.12 It plays a role in the amplification of inflammatory responses induced by TLR ligands.17 The role of IL-6 has recently been confirmed by the therapeutic effects of tocilizumab (TCZ), a neutralising antibody to the IL-6 receptor (IL-6R).18 However, some research shows that serum IL-6 levels in patients with MAS did not differ from IL-6 levels during active SJIA in the absence of MAS.1
IL-18 is a unique cytokine in the IL-1 family and is constitutively present in keratinocytes, epithelial cells, and blood monocytes. It induces production of IFN-γ by NK cells and T cells as well as TNF-α and chemokine secretion by macrophages.16 Some reports showed that an imbalance between IL-18 and its natural inhibitor, IL-18-binding protein, resulted in Th-1 lymphocyte and macrophage activation, which escaped the control by NK-cell-mediated cytotoxicity and may have allowed for secondary hemophagocytic syndrome.19
IFN-γ is a proinflammatory cytokine produced by T lymphocytes and NK cells when activated by antigen-presenting cells via IL-12 and IL-18. The primary function is to profoundly activate monocytes and macrophages.16 The high levels of IFN-γ and chemokine (C-X-C motif), ligand 9 (CXCL9), CXL10, and CXCL11 and their correlation with the severity of laboratory abnormalities of MAS suggest a pivotal role of IFN-γ in MAS.20
TNF-α is produced largely by monocytes and macrophages that are activated by TLR ligands, such as endotoxin, and cytokines, such as IL-18, stimulating local endothelial cells as well as lymphocytes. TNF-α similarly does not appear to be central to the pathogenesis of MAS in patients with SJIA. Serum levels of TNF-α are only modestly elevated and are not significantly higher than those seen in active SJIA.16
DIAGNOSTIC CRITERIA OF MACROPHAGE ACTIVATION SYNDROME
MAS associated with JIA was first described by Hadchouel et al.2 The diagnosis of MAS is often challenging, since the disease can mimic infections and malignancies, and presents as a life-threatening complication with SJIA. Ravelli et al.21 made a preliminary diagnostic guideline for MAS complicating SJIA in 2005 (Table 1). The diagnosis of MAS requires the presence of ≥2 of the following laboratory criteria, or ≥2 of the following clinical criteria:
- Ravelli et al’s.21 criteria have been applied over many years, and some clinicians hold the view that the criteria are of value only in patients with active SJIA. The thresholds of laboratory criteria are provided only as an example. The clinical criteria are probably more useful as classification criteria rather than as diagnostic criteria because they often occur late in the course of MAS; therefore, they may hold limited value in the early diagnosis of the syndrome.2
- In 2013, the American College of Rheumatology (ACR) updated the 2011 recommendations for the treatment of juvenile idiopathic arthritis, focussing especially on the addition of SJIA recommendations. They focussed on SJIA with features that are concerning for MAS. The features concerning for MAS were defined as any combination of the following disease manifestations: persistent (rather than quotidian) fever, cytopenias or falling cell line counts (particularly platelets), falling erythrocyte sedimentation rate, hypertriglyceridaemia, hypofibrinogenaemia, haemophagocytosis, transaminitis, coagulopathy, organomegaly, low or absent NK cell activity, hyperferritinaemia, or central nervous system dysfunction. This definition was left intentionally broad given the lack of validated classification criteria for MAS. The scenarios specifically excluded critically ill patients requiring intensive care unit admission.22
- In 2016, the European League Against Rheumatism (EULAR)/ACR approved classification criteria for MAS complicating SJIA, after quantitatively validating the criteria in patient datasets. Experts identified the laboratory tests (platelet count, and serum ferritin and aspartate aminotransferase levels) in which changes over time are most valuable for the timely diagnosis of MAS occurring in the context of SJIA.23 They found that ferritin levels (≥500 ng/mL) could discriminate between MAS and systemic infections. Hyperferritinaemia did not increase the sensitivity and specificity of the guidelines for MAS in SJIA, but it did enhance their capacity to differentiate MAS from systemic infections.24 According to the criteria, a febrile patient with known or suspected SJIA is classified as having MAS if several criteria are met (Table 2).25
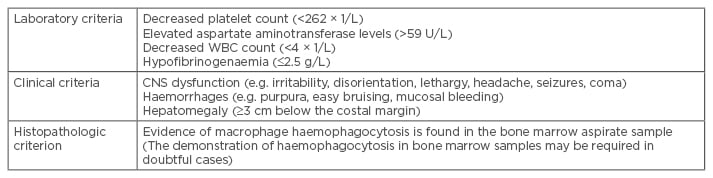
Table 1: Preliminary diagnostic guidelines for macrophage activation syndrome complicating systemic
juvenile idiopathic arthritis in 2005.21
WBC: white blood cell count; CNS: central nervous system.

Table 2: EULAR/ACR criteria for MAS complicating SJIA in 2016.25
EULAR: European League Against Rheumatism; ACR: American College of Rheumatology; MAS: macrophage activation syndrome; SJIA: systemic juvenile idiopathic arthritis.
Differential Diagnosis
Many conditions can lead to the clinical picture of MAS, including malignancies (leukaemia, lymphoma, other solid tumours), infections (viral, bacterial, or parasitic), and rheumatoid disorders (systemic lupus erythematosus/Kawasaki disease). MAS, a serious complication of systemic inflammatory disorders, is thought to be caused by excessive activation and proliferation of T lymphocytes and macrophages; moreover, in the same way as in HLH, it has been shown that in addition to corticosteroids, cyclosporin A (CsA) is also effective in patients with MAS.11
TREATMENT
In the treatment of MAS, extensive, high dose corticosteroid treatment is used in the beginning to control the findings. In addition, CsA treatment is used as immunosupressive treatment. Etoposide treatment is used in primary HLH. It is rarely used in cases of MAS related with SJIA. Intravenous immunoglobulin treatment is currently applied in some cases.15 In maintenance treatment, or in rare cases where activity findings cannot be suppressed with classical treatment, anti-IL-1 or IL-6 treatment is used. Anakinra (IL-1 alpha and beta inhibition), canakinumab (IL-1 beta inhibition), and TCZ (IL-6 inhibition) are biological drugs used for this purpose.3,26
Non-Biologic Treatments
In treatment, corticosteroids and cyclosporin are used.22 Most clinicians start with intravenous methylprednisolone pulse therapy (e.g. 30 mg/kg for 3 consecutive days) followed by 2–3 mg/kg/day in 2–4 divided doses. If response to steroids is not immediately evident, parenteral administration of CsA (2–7 mg/kg/day) is usually initiated. Addition of CsA not only provides rapid control of the symptoms, but also avoids excessive use of steroids.16 Etoposide was reported to be used in MAS because it is a central medication in HLH therapeutic protocols and it was able to induce rapid recovery in cases of steroid and CsA resistant MAS without serious adverse events.15,23 The potential toxicity of the drug etoposide is a major concern, particularly in patients with hepatic impairment.16 It has been argued that the HLH protocol is too aggressive for use as first-line therapy in SJIA–associated MAS.15
Biologic Agents
Anti-IL-1 drugs are used in some resistant cases.23 For severely ill children, IL-1 blockade has been remarkably effective in a relatively brief time frame, such as anakinra (IL-1 alpha and beta inhibition), canakinumab (IL-1 beta inhibition). Rituximab, an anti-CD20 antibody that depletes B lymphocytes, has been successfully used in Epstein–Barr virus-induced lymphoproliferative disease and could be considered in Epstein–Barr virus-driven MAS.16
IL-6 blockade, via the anti-IL-6 receptor monoclonal antibody TCZ, has proven highly efficacious in treating SJIA.16 The role of IL-6 has recently been confirmed by the therapeutic effects of TCZ,18 but the additional caution in TCZ use for children with a history of MAS is warranted to avoid serious adverse events related to cytopenias.27 Given that infections remained the most prevalent trigger for MAS in canakinumab-treated patients with SJIA, increased vigilance and prompt initiation of aggressive therapy appear warranted in al suspected cases.28
CONCLUSION
MAS is a complication of SJIA which increases morbidity and mortality and can be rapidly fatal
if left untreated. It requires prompt recognition to initiate appropriate treatment and prevent
deleterious outcomes.23 Early detection and early intervention are vital to avoiding poor consequences.






