Abstract
JAK inhibitors (JAKi) are targeted, small-molecule, disease-modifying therapies that are the newest class of treatments to emerge for the management of rheumatoid arthritis (RA) and the first oral disease-modifying anti-rheumatic drugs (DMARD) to demonstrate comparable clinical efficacy to biological DMARDs (bDMARD). In the UK there are four JAKi licensed for the treatment of RA (baricitinib, tofacitinib, upadacitinib, and filgotinib) and recent years have seen an explosion in their use. Clinical trial evidence supports their efficacy in a range of RA cohorts including DMARD-naïve patients and those with treatment-refractory disease. JAKi are associated with increased risk for infection, particularly herpes zoster virus reactivation, cytopenias, and hyperlipidaemia. In older patients with cardiovascular risk factors, post-marketing data suggest increased risk for malignancy, venous thromboembolism (VTE), and major cardiovascular events (MACE) with JAKi. This review article discusses the mechanism of action of JAKi and the evidence for their efficacy and side effect profile.
Key Points
1. JAK inhibitors (JAKi) have gained an important role in the management of rheumatoid arthritis, and current clinical guidance recommends their use in patients who have shown an inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (csDMARD).2. Current evidence suggests that JAKi are at least as efficacious as the previous standard of care therapies and that they are effective in a range of patient subtypes, including those with difficult-to-treat disease.
3. JAKi pose an increased risk of infection and there is concern that they increase the risk of malignancy, venous thromboembolism, and major cardiovascular events in certain patient groups; further research is needed to characterise this.
INTRODUCTION
JAK inhibitors (JAKi) are the latest class of targeted, disease-modifying therapies licensed for the treatment of rheumatoid arthritis (RA). This review article discusses the role of JAKi in RA and their mechanism of action, before considering the evidence of their efficacy and adverse events.
RA is a chronic systemic inflammatory condition which, without early and effective treatment, results in progressive, erosive arthritis with pain, loss of physical function, joint deformity, and deterioration in quality of life.1 Since the 1990s, the cornerstone of treatment has been conventional synthetic disease-modifying anti-rheumatic drugs (csDMARD), with methotrexate commonly prescribed as first-line.1 Many patients, however, may discontinue methotrexate therapy due to inadequate response (IR) to treatment, secondary loss of response, or the development of adverse effects (AE).1,2 Beyond methotrexate, the past 20 years have seen the development and approval of more targeted treatments, including biologic DMARDs (bDMARDs) such as TNF inhibitors (TNFi); IL-6 and IL-1 inhibitors; anti-CD20 monoclonal antibodies; and cytotoxic T-lymphocyte antigen-4 inhibitors.2 Despite these developments, only 40–50% of patients achieve disease remission.2,3 Therefore, there remains an unmet need for RA management in terms of treatment tolerability and optimal disease control.2,3
JAK INHIBITORS AND THEIR MECHANISM OF ACTION
The latest class of drugs used in the treatment of RA are the JAK inhibitors (JAKi). JAKi are selective, small-molecule oral DMARDs that inhibit cytokine signal transduction via the JAK-signal transducers and activators of transcription (STAT) pathway.1 There are four main JAK isoforms (JAK1, JAK2, JAK3, and tyrosine kinase 2), to which each JAKi exerts variable molecular selectivity, as summarised in Table 1.1,2 As illustrated in Figure 1, JAKs exist with their associated STAT proteins to exert signal transduction.1 JAK-STAT signalling is initiated when cytokines bind their cognate receptors on the extracellular surface membrane. This induces a conformational change in the receptor and the recruitment and activation of associated JAKs by phosphorylation.1,2 Activated JAKs thereon auto-phosphorylate residues on the intracellular domains of the cytokine receptor, acting as docking sites for associated STAT proteins.1 JAKs also phosphorylate STATs, which dissociate from their docking sites and dimerise to form phosphorylated STAT–STAT dimers. These translocate to the nucleus and bind to specific DNA regions, initiating gene transcription and hence protein translation.1 Therefore, the net effect of the JAK-STAT signalling pathway is to stimulate gene expression in response to extracellular ligands. Different cytokines are dependent on different JAK and STAT proteins. This is summarised in Figure 1 and forms the theoretical basis for the development of isoform-specific inhibitors.
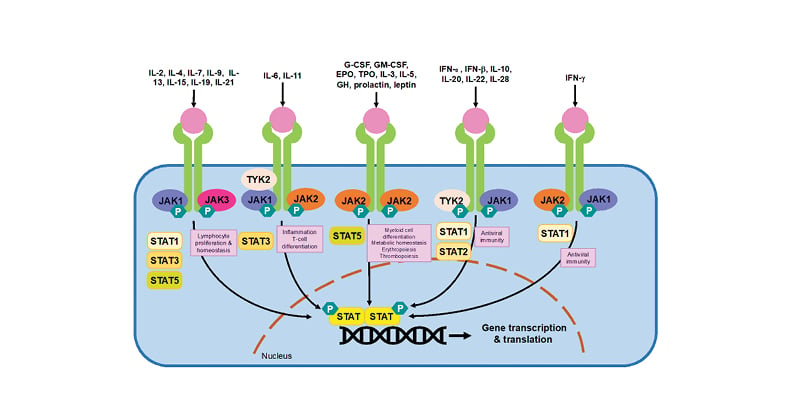
Figure 1: The JAK-STAT signal transduction pathway.
Adapted from Winthrop et al.68 and Harrington et al.1
JAKs are activated when ligands, such as cytokines or growth factors, bind to their cognate receptor. JAK activation results in the recruitment, phosphorylation and dimerisation of STATs. STAT dimers translocate to the nucleus and stimulate gene transcription. As illustrated, different cytokine receptors preferentially use different JAK and STAT proteins to signal.
IL: interleukin, JAK: janus kinase, STAT: signal transducer and activator of transcription, TYK: tyrosine kinase, G-CSF: granulocyte colony-stimulating factor, GM-CSF: granulocyte-macrophage colony-stimulating factor, EPO: erythropoietin, TPO: thrombopoietin, GH: growth hormone, IFN: interferon, P: phosphorylation.
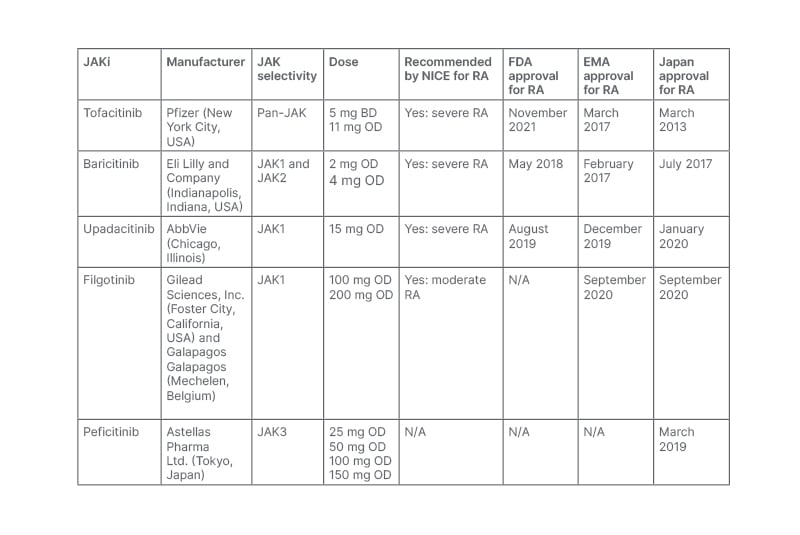
Table 1: A summary of JAK inhibitors’ selectivity, dosing, and current National Institute for Health and Care Excellence (NICE), U.S. Food and Drug Administration (FDA), and European Medicines Agency (EMA) approval status in RA.
BD: twice daily; FDA: U.S. Food and Drug Administration; JAK: janus kinase; JAKi: janus kinase inhibitor; NICE: National Institute for Health and Care Excellence Clinical; OD: once daily; RA: rheumatoid arthritis; TEC: tyrosine kinase expressed in hepatocellular carcinoma.1
JAK inhibitors IN RHEUMATOID ARTHRITIS
In November 2012, tofacitinib became the first U.S. Food and Drug Administration (FDA)-approved JAKi for patients with moderate-to-severe RA with intolerance or IR to methotrexate.4 Targeting the JAK pathway gained further pharmaceutical interest, resulting in the development of baricitinib in May 2018 and upadacitinib in August 2019.1 In the UK, tofacitinib, baricitinib, and upadacitinib have all been recommended by the National Institute for Health and Care Excellence (NICE) for the treatment of severe RA, and in February 2021 filgotinib became the first JAKi to become licensed for patients with moderate RA.5 These recommendations have resulted in the widespread prescription of JAKi in the UK and worldwide. Throughout this review article, the authors review the latest data evaluating their efficacy and safety, focusing on tofacitinib, baricitinib, upadacitinib, and filgotinib.
EVIDENCE FOR THE EFFICACY OF JAKi IN RA
Clinical trial evidence strongly supports the efficacy of JAKi in the management of RA. In these studies, JAKi have been shown to significantly improve a range of RA-related outcomes, including disease activity, patient function, radiographic progression, and patient-reported outcome measures. Clinical efficacy has been demonstrated in a range of patient groups, including treatment of naïve patients and those with IR to csDMARDs and bDMARDs. Throughout the next section, the authors review the randomised controlled trial (RCT) evidence to support the use of the four JAKi licensed for the treatment of RA in the UK: tofacitinib, baricitinib, filgotinib, and upadacitinib. The major studies supporting their clinical effectiveness are outlined in Table 2.
Tofacitinib was the first JAKi to demonstrate clinical efficacy in the treatment of RA.6 In the landmark ORAL Solo trial, tofacitinib demonstrated superiority over placebo in patients who were methotrexate-naïve with respect to American College of Rheumatology (ACR) response criteria, Health Assessment Questionnaire-Disability Index scores, and remission rates.7 More recently, baricitinib, filgotinib, and upadacitinib have all demonstrated clinical efficacy in RCTs of participants with RA.8-13
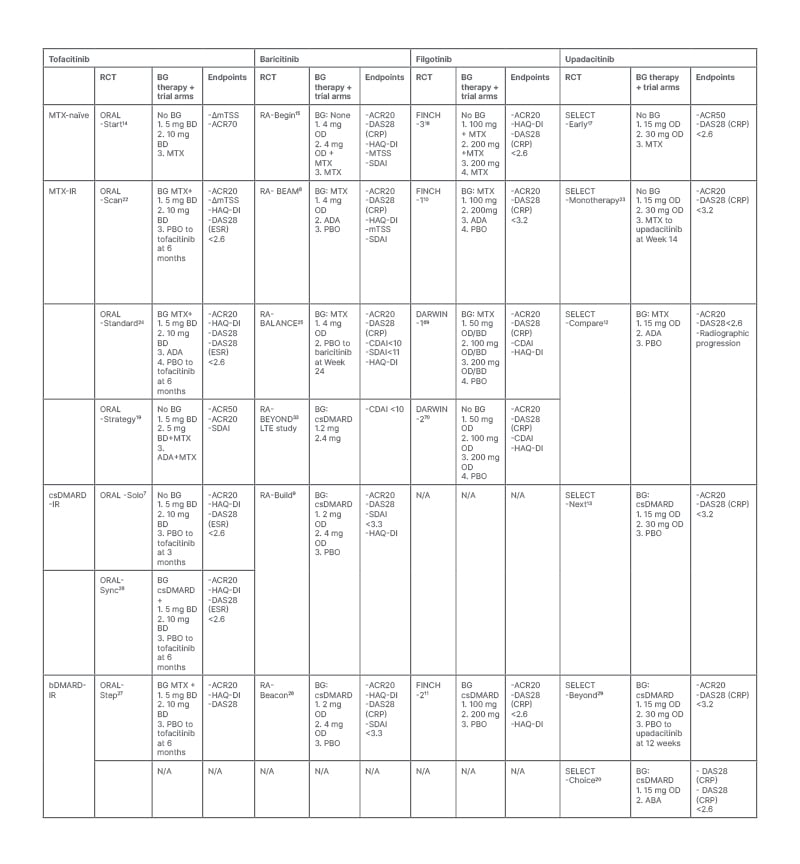
Table 2: Key randomised control trials providing evidence for the efficacy of JAK inhibitors in the treatment of rheumatoid arthritis in a range of patient subtypes.
BD: twice daily; FDA: U.S. Food and Drug Administration; JAK: janus kinase; JAKi: janus kinase inhibitor; NICE: National Institute for Health and Care Excellence Clinical; OD: once daily; RA: rheumatoid arthritis; TEC: tyrosine kinase expressed in hepatocellular carcinoma.1
Many effective treatments have been long-established in the management of RA. Therefore, head-to-head trials comparing JAKi to standard of care therapy were essential prior to clinical licensing. Current evidence suggests that JAKi are more effective than csDMARDs in some cases, with tofacitinib, baricitinib, and upadacitinib demonstrating superiority compared to methotrexate in RCTs.14-17 In contrast, filgotinib did not produce superior ACR response rates compared to methotrexate in the FINCH3 RCT.18
Before the licensing of JAKi, bDMARDs were well-established as the gold standard treatment for RA, with their use preserved for patients with a IR to csDMARDs. As increasing evidence supporting the efficacy of JAKi emerged, the question as to whether they could be as efficacious as bDMARDs arose. This led to the planning and execution of four pivotal head-to-head RCTs, all of which suggested that JAKi were at least as efficacious as the TNFi adalimumab.8,10,12,19 These trials included the ORAL-STRATEGY and FINCH-1 trials, which demonstrated non-inferiority of tofacitinib and filgotinib to adalimumab in the management of RA.10,19 The RA-BEAM trial was a pivotal head-to-head study that compared baricitinib and adalimumab in participants with RA with IR-methotrexate.8 In addition to demonstrating non-inferiority compared to adalimumab, baricitinib was superior with respect to ACR20 response rate and disease activity measured using the Disease Activity Score (DAS)-28.8 RA-BEAM was the first RCT to demonstrate superiority of JAKi to biological medications, and led to the rapid uptake of these medications in clinical practice. More recently, upadacitinib has also demonstrated superiority compared to bDMARDs, including adalimumab and the cytotoxic T-lymphocyte antigen-4 inhibitor abatacept.12,20 There have been no head-to-head trials comparing JAKi to other classes of biologics such as IL-6 or CD20 inhibitors.
DIFFICULT TO TREAT RHEUMATOID ARTHRITIS
The aforementioned evidence supports the clinical efficacy of JAKi in RA, and suggests that they are at least as efficacious as the current standard of care. Importantly, clinical trial data also demonstrates the effectiveness of JAKi in patients with refractory disease who have shown IR to csDMARDs and bDMARDs. This is an important group to consider, as a significant proportion of patients with RA do not respond to first- or second-line therapies.21
Table 2 summarises the RCTs investing the efficacy of JAKi in patients with RA who are methotrexate-naïve and those with IR-methotrexate, IR-csDMARDs, and IR-bDMARDs. Notably, there is an abundance of Phase III evidence supporting the efficacy of JAKi in patients with IR to csDMARDs and methotrexate.7-10,12,13,19,22-26 Furthermore, tofacitinib, baricitinib, upadacitinib, and filgotinib have also demonstrated clinical efficacy in patients who have previously received at least one bDMARD.11,27-29 Registry data from a Japanese cohort of patients with RA with difficult to treat disease, defined as previous IR to two or more bDMARDs or targeted synthetic DMARDs, found that JAKis were associated with the highest proportion of rapid responders and the best outcome in clinical disease activity index in comparison to other bDMARDs or targeted synthetic DMARDs.30 Overall, these data suggest that JAKi can play important roles as second-, third-, or fourth- line therapies in patients who have failed previous treatments, and emerging data suggest that they may be preferable in this setting.
ADDITIONAL CONSIDERATIONS
JAKi In Combination or Alone?
Current guidelines support the administration of JAKi alongside csDMARD therapy. This is in accordance with RCT evidence suggesting that JAKi are more efficacious in combination than as monotherapy. For example, in the ORAL-STRATEGY trial, treatment with tofacitinib and methotrexate was associated with increased rates of ACR response, low disease activity, and clinical remission, compared to tofacitinib monotherapy.19 Superior outcomes with combination therapy have also been demonstrated in trials of baricitinib and filgotinib.15,18
Initial Dosing of JAKi
Current clinical guidelines advise that JAKi doses may be adjusted with increased age, or with liver or renal impairment.31 In the absence of these exceptions, patients are generally commenced on standard doses of JAKi (summarised in Table 1). As illustrated in Table 2, RCTs have investigated the relative efficacy of different doses of JAKi. Some of these studies suggest that the higher of the licensed doses are associated with increased clinical efficacy; however, this should be balanced against the possibility of increased risk of adverse events, which will be discussed later.10 In contrast, other data suggest no difference in efficacy between doses. For example, a meta-analysis showed no difference in clinical outcomes including the ACR20, DAS-28, and Health Assessment Questionnaire-Disability Index, between the licensed doses of baricitinib, tofacitinib, and upadacitinib.32
Tapering of Doses?
In patients with RA on long-term JAKi, clinical guidance suggests that the dose may be tapered when clinical remission is achieved.31 The evidence for this is lacking, but has been investigated in patients on baricitinib in the RA-BEYOND trial. This trial studied patients with RA on baricitinib with low disease activity (LDA), or in remission.33 In this trial, participants were randomised to continue at a 4 mg daily dose, or to reduce to 2 mg. This study demonstrated that dose reduction was associated with a small but significant fall in those sustaining LDA or remission. Whilst there was a higher risk of relapse in the group taking 2 mg baricitinib (37% versus 23%; p=0.001), most patients on the lower dose maintained LDA.33 Furthermore, patients who were weaned to 2 mg could recapture remission if returned to 4 mg daily.33 Therefore, these data suggest that most patients on long-term JAKi therapy maintain LDA when weaned to lower doses. If relapse occurs, LDA can usually be recaptured by increasing the dose.33
Predictors of Response to JAKi
As an increasing number of therapies are licensed for RA, predictors of treatment response have gained interest. Post-hoc analysis of five Phase III studies found that patients with RA with positivity for anti-cyclic citrullinated peptide and rheumatoid factor were more likely to achieve ACR 20/50/70 responses than seronegative patients.34 DAS-28 remission rates and quality of life measures were also lower in anti-cyclic citrullinated peptide-negative patients.34 There were no other significant differences between achievement of endpoints in seronegative versus seropositive patients.34 Future work is needed to confirm this finding and to explore other markers of treatment response.
Comparative Efficacy of JAKi
The relative efficacy and safety of different JAKi is an area of uncertainty, and there have been no head-to-head comparison studies in this area. Indirect comparison using meta-analyses of RCT data have attempted to characterise the differences between JAKi. Due to study heterogeneity, these indirect analyses must be interpreted with caution, and conclusions can be misleading.
Lee et al.31 performed a network meta-analysis to evaluate the comparative efficacy and safety of tofacitinib, baricitinib, upadacitinib, filgotinib, and perficitinib as monotherapy in individuals with RA. Five RCTs with a total of 1,547 patients were included in the analysis.31 The analysis found that all five JAKi were associated with a significantly higher ACR20 response rate than placebo. Peficitinib 150 mg was found to be the most efficacious JAKi, measured using the probability of achieving the ACR20.31 Peficitinib 150 mg was followed by pefictinib 100 mg, filgotinib 200 mg, filgotinib 100 mg, tofacitinib 5 mg, upadacitinib 12 mg, and baricitinib 4 mg in achieving ACR20. ACR50 and 70 response rates showed similar patterns.31
In contrast with the results from Lee et al.,31 two meta-analyses have suggested that upadacitinib is the most efficacious JAKi.32,35 Pope et al.35 reported that upadacitinib was more effective when compared to tofacitinib and baricitinib. Furthermore, Weng et al.32 found that upadacitinib was the most efficacious JAKi in a meta-analysis comparing the relative efficacy of csDMARDs, bDMARDs, and JAKi in patients with RA with IR to at least one csDMARD.32 In this study, 88 studies and 31,566 patients were included. bDMARDs and JAKi were more efficacious than placebo in all three measures of drug efficacy.32 Whilst upadacitinib was the most efficacious JAKi, the IL-6 inhibitor tocilizumab was the most efficacious medication in improving DAS-28 scores.32
Real-world studies have also investigated the relative efficacy of JAKi by analysing registry data. In one such study, baricitinib was shown to demonstrate significantly better clinical outcomes, measured using clinical disease activity index, and similar safety profiles when compared to tofacitinib.36 These data should be interpreted with caution due to a small sample size (n=294).36
In summary, there is an abundance of evidence supporting the clinical efficacy of JAKi in the treatment of RA. Current evidence suggests that JAKi are at least as efficacious as the previous standard of care therapies and that they are effective in a range of patient subtypes, including those with difficult-to-treat disease. Trial evidence supports a beneficial effect of combination therapy, with guidance suggesting that JAKi should be prescribed alongside csDMARDs. The major outstanding area of uncertainties relate to the comparative efficacy between individual JAKi, and between JAKi and bDMARDs.
Side Effects and Safety Profile
JAKi have been associated with a broad range of side effects, summarised in Table 3. Most notably, increased risk of infection, malignancy, venous thromboembolism (VTE), and major cardiovascular events (MACE) have been described in patients receiving JAKi. The evidence for this will be summarised in the next section of the review.
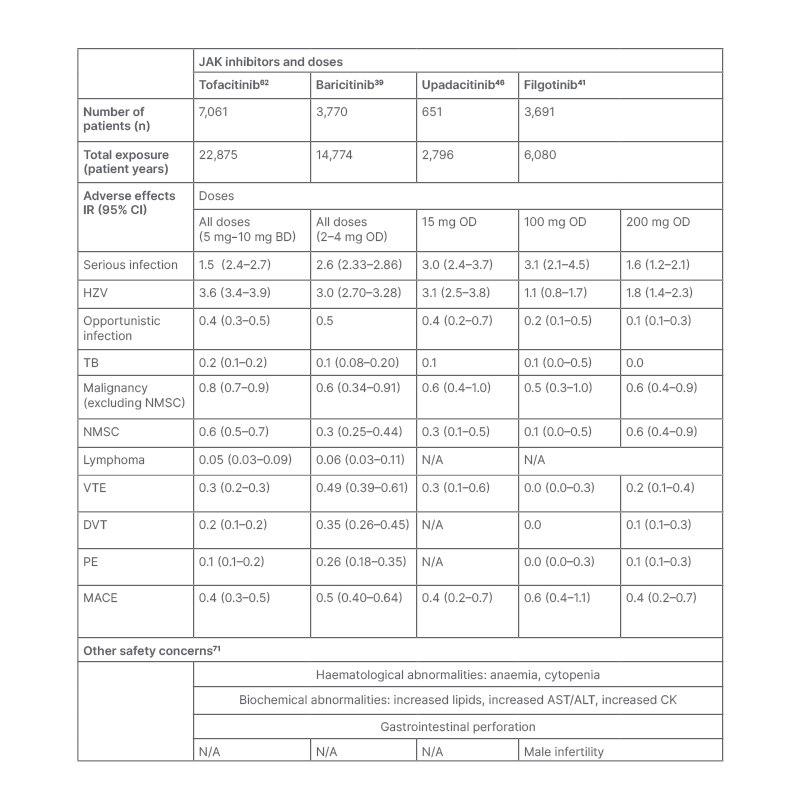
Table 3: A summary of the common adverse events from four long-term integrated safety analyses of four JAK inhibitors in the treatment of rheumatoid arthritis. Other safety concerns are summarised in the lower half of the table.
ALT: alanine aminotransferase; AST: aspartate aminotransferase; BD: twice daily; CI: confidence interval; CK: creatine kinase; DVT: deep vein thrombosis; HZV: herpes zoster virus; InR: incidence rate: MACE: major adverse cardiovascular event; N/A: not applicable; NMSC: non-melanoma skin cancer; OD: once daily; PE: pulmonary embolus; TB: tuberculosis; VTE: venous thromboembolism.
Infection
In similarity to other immunomodulatory therapies, the most commonly reported AEs with JAKi are infections.37 The risk of infection with JAKi has been evaluated in Phase II, Phase III, and long-term extension (LTE) studies, with incident rate (InR) estimates ranging between 1.6 and 3.0 per 100 patient-years (PY) for those on JAKi.38-41 In these studies, the rates of serious infection are stable over time with pneumonia being the most commonly reported.42 In studies of JAKi, risk factors for infection include age, steroid usage (prednisolone ≥7.5 mg/day), disease activity, diabetes, and higher dosage (10 mg twice daily [BD] versus 5 mg BD).42-44 Similar rates of infection have been reported in patients receiving baricitinib, upadacitinib, and filgotinib, with meta-analyses demonstrating no significant difference in infection risk between JAKi.40,41,45,46
The relative risk for infection in patients taking JAKi seems comparable to those taking bDMARDs, with one retrospective cohort study reporting no significantly increased risk of serious infection with tofacitinib compared with TNFi.47 Similar rates of infection in participants taking JAKi and bDMARDs have also been demonstrated in head-to-head RCTs and in post-approval registries.8,10,12,24,48 In elderly patients, the German prospective register RABBIT reported that csDMARDs, bDMARDs, and JAKis were associated with similar rates of infection.44 Whether there are differences in the pattern of bacterial infection between JAKi and bDMARDs remains an area of uncertainty.49
Herpes Zoster
The reactivation of herpes zoster virus (HZV) is a widely recognised complication of JAKi, with trial data showing a greater risk of HZV with JAKi compared to placebo, csDMARDs, and bDMARDs.49 In Phase II, Phase III, and LTE studies, the IR of HZV ranges between 1.1 and 3.6 per 100 PY for those on JAKi.38-41 Rates of HZV are higher in Asian countries, including Japan (IR=8.0 per 100 PY) and Korea (IR=8.4 per 100 PY); however, the reasons for this are unclear.38 In studies of tofacitinib, significant risk factors for HZV include age, corticosteroid use, co-prescription of methotrexate, smoking, and higher JAKi dose.38
As summarised in Table 3, the rates of HZV reactivation appear similar between tofacitinib, baricitinib, and upadacitinib.39-41 Pooled data evaluating JAK1 selectivity suggest that filgotinib is associated with fewer HZV infections; however, further data are required to conclude this.49 In comparison with bDMARDs, HZV is seen significantly more frequently with JAKi. In one study, the crude IR for HZV in patients with RA receiving tofacitinib was 3.87 per 100 PY (95% confidence interval [CI]: 2.92–5.32), compared with 1.95 per 100 PY in patients on adalimumab (95% CI: 1.65–2.31).50 This has also been reported in post-approval registry studies, including the American CorEvitas register, where the hazard ratio for HZ reactivation was 2.32 (95% CI: 1.43–3.75) for tofacitinib versus bDMARDs.48
Malignancy
The overall risk of malignancy in patients with RA is moderately elevated when compared to the general population.51 There is concern that JAKi increase this risk further by preventing the immune-mediated elimination of cancerous cells through decreased interferon production and reduced circulating natural killer cells.37,52 Pooled data from Phase II, Phase III, and LTE studies found 107 of 5,671 patients with RA treated with tofacitinib developed malignancies (excluding non-melanoma skin cancers) with the commonest being lung (n=24), breast (n=19), lymphoma (n=10), and gastric cancer (n=6).53 The rate of malignancy was stable at 6-month intervals and comparable to that seen in the general RA population.53 Similar rates have been reported with baricitinib, upadacitinib, and filgotinib.39-41 In similarity to bDMARDs, the risk of non-melanoma skin cancers may be raised with JAKi; however, the evidence is not clear.54
A systematic review and meta-analysis of RCTs and LTEs concluded that tofacitinib showed no significantly increased risk of malignancy in patients when compared with those receiving csDMARDs or placebo.55 In contrast, the ORAL Surveillance post-authorisation trial found that tofacitinib was associated with increased risk of cancer, when compared with a TNFi, in a cohort of older patients.56 It is unclear whether the risk of malignancy was increased with JAKi or decreased with TNFi. Nevertheless, the FDA issued a warning for the use of tofacitinib and JAKi in the elderly population, and TNFi may be preferable in this cohort, pending further clarity.53,61,62
Thromboembolic Events
Immune-mediated inflammation, as occurs in RA, is a risk factor for VTE (including pulmonary embolism and deep venous thrombosis). A nationwide register-based cohort study found that patients with RA were 1.88 times more likely to develop VTE than those without.57 In this study, disease activity was a significant risk factor for VTE, with a two-fold increase in risk in patients with disease remission compared to high disease activity.57
There has been concern that JAKi are independent risk factors for VTE, with some trial and post-marketing evidence suggesting increased risk with baricitinib and tofactinib.58 Although the interpretation of these data is limited by small event numbers, the labelling for tofacitinib, baricitinib, upadacitinib, and filgotinib list thrombosis as a warning and clinicians are advised to use with caution in those with underlying risk factors.59-61 In Phase II, Phase III, and LTE studies of patients with RA receiving JAKi, IR estimates for VTE range between 0.17 and 0.49 per 100 PY, with rates stable over time, similar between JAKi subclasses, and higher in those with background thrombotic and cardiovascular risk factors.39-41,60,62 Long-term safety data for tofacitinib suggest that the risk for VTE is dose-dependent, with increased rates in those taking 10 mg BD, a dose that is licensed for ulcerative colitis and not RA.43 This was also demonstrated in the ORAL surveillance study, where a higher risk for VTE was seen with tofacitinib 10 mg BD versus TNFi, but not with 5 mg BD.56
In contrast to current warnings, a meta-analysis of 42 RCTs found no increased risk for VTE in those receiving JAKi compared to placebo.61 Furthermore, real-world evidence using registry data from >85,000 patients with RA receiving JAKi or TNFi showed no evidence for increased risk for VTE with tofacitinib.57 These results are reassuring but should be interpreted with caution in patients with underlying risk factors for VTE, in whom JAKi are generally avoided.61
Major Adverse Cardiovascular Events
Patients with RA have an approximately 70% higher risk of cardiovascular (CV) disease compared to the general population.63 Safety data are generally reassuring and suggest that the observed MACE IR ranges between 0.4 and 0.6 per 100 PY in those treated with JAKi, which is comparable to the general RA population.41,46,64 In contrast, the ORAL Surveillance study found that the incidence of MACE were higher with tofacitinib compared to TNFi (hazard ratio: 1.33; 95% CI: 0.91–1.94).56 In older patients with at least one CV risk factor, tofacitinib was associated with more MACE compared to TNFi.56,65,66 These results were extrapolated to all JAKi, and in September 2021 the FDA released an updated black box warning regarding increased risk of death, CV disease, malignancies, and thrombosis in those on JAKi versus TNFi.56,65,66 As TNFi are known to decrease the risk of MACE in RA cohorts, the results from the ORAL surveillance study could suggest that TNFi are relatively more protective against MACE than JAKi.34 Whilst further work sheds insight in this area, TNFi may be preferable to JAKi in patients with RA with pre-existing CV risk factors.
SUMMARY
JAKi have gained an important role in the management of RA, and current clinical guidance recommends their use in patients who have shown a IR to csDMARDs. RCTs to date support the efficacy of JAKi in a range of patient groups, but there remain outstanding areas of uncertainty. Most notably, the comparative efficacy and safety of the different JAKi remains unclear, and meta-analyses in this area have produced conflicting results. Although the different JAKi exert differential isoform selectivity, differences between JAKi in efficacy or AEs have not been observed in trial or real-world data. Furthermore, whilst head-to-head trials have compared the efficacy of JAKi to TNFi, there have been no head-to-head studies characterising the relative efficacy of JAKi compared to bDMARDs such as IL-6 inhibitors. In those who have received JAKi, there is uncertainty as to whether switching between JAKi is effective following IR to primary JAKi. Overall, further work is needed to characterise the predictors of response to JAKi, in addition to csDMARDs and bDMARDs, with personalised medicine being the ultimate aim in the treatment of RA.
In addition to uncertainties regarding efficacy, the long-term safety of JAKi is relatively unclear, and there are ongoing safety concerns. Most notably, tofacitinib has been associated with increased cardiovascular disease and cancer, in comparison to TNFi, in older adults.56 Other areas of uncertainty include the risk of testicular toxicity with filgotinib,67 the risk of VTE in patients with thrombotic risk factors, and the relative safety of the different JAKi. Future work is needed to address these issues and confirm which patient groups can be given JAKi without significant risk of adverse outcomes.





