Abstract
Food allergy is potentially life-threatening and has a major impact on quality of life. Avoidance is currently the only approved therapy, and, although effective, avoidance diets can be difficult and may also put children at risk of nutritional deficiencies and impaired growth. At least 80% of milk and egg-allergic children are expected to achieve natural tolerance to these foods by adulthood, and 15–20% of peanut or tree nut-allergic individuals ‘outgrow’ their allergies. Effective therapies for food allergies are therefore highly desirable. There have been several immunotherapies for food allergy such as oral immunotherapy (OIT), sublingual immunotherapy (SLIT), epicutaneous immunotherapy (EPIT), and OIT combined with anti-IgE monoclonal antibodies (omalizumab [OMB]). However, efficacy and safety have only been demonstrated in one large Phase III trial for peanut allergies. Additionally, there have only been three randomised, controlled studies of OMB–OIT combination and these were low-powered, single-centre trials; therefore, evidence levels were low in these trials. Studies that included long-term follow-up observations and clinical tolerance are rare. Additionally, clinical tolerance is not well-defined and remains unknown. Therefore, several problems remain to be resolved, but hopefully OIT in combination with OMB will resolve these problems in the future. Although there are only three randomised, controlled trials of OMB–OIT, the combination therapy enabled high dose desensitisation for a short duration without any adverse events, resulting in the sustained unresponsiveness in IgE-related food allergy. It is speculated that this combination therapy will be the most effective immunotherapy in the future.
INTRODUCTION
The prevalence of IgE-mediated food allergy is increasing in Western and developed countries,1 and the most common food allergies are milk, egg, wheat, peanut, tree nuts, soy, fish, shellfish, and sesame.2 Treatment of food allergies has been carried out on strict avoidance of the offending food(s) and is performed when accidental ingestions occur. Food allergies are potentially life-threatening and have a major effect on the quality of life.3,4 Avoidance is currently the only approved therapy for food allergies, and although avoidance diets are effective, they can be difficult and may also put children at risk of nutritional deficiencies and impaired growth.5-7 At least 80% of milk and egg-allergic children are expected to achieve natural tolerance to these foods by adulthood, and 15–20% of peanut or tree nut-allergic individuals outgrow their allergies.7 Therefore, effective therapies for food allergies are highly desirable. There have been several immunotherapies for food allergies, such as oral immunotherapy (OIT), sublingual immunotherapy (SLIT), epicutaneous immunotherapy (EPIT), and OIT combined with anti-IgE monoclonal antibodies (omalizumab [OMB]; OMB–OIT). This review focusses on the advantages, disadvantages, and differences in these immunotherapies, especially OMB–OIT therapy.
ORAL IMMUNOTHERAPY
OIT as an active intervention for food allergies has been mainly performed in Europe, USA, and Japan.8 OIT for eggs, milk, wheat, and peanuts has been reported in these countries;8,9 however, OIT is not recommended as a general practice because of problems with safety.10 Considering that there have been many recent outstanding reviews on individual OIT studies,10-15 in this review, the authors will briefly focus on a general overview of OIT, current challenges, and clinical trials that are in progress.
With OIT, patients ingest food daily between dose escalations. Notably, there is a wide range of dosing protocols in terms of the build-up and maintenance phases. In peanut trials, studies have used maintenance doses ranging from 1 peanut (approximately 200 mg) to 17 peanuts. Furthermore, there have been significant differences in the reporting of outcomes, which are likely related to varied definitions used in differing studies. These differences could also be attributable to the effects of the time and the dose of therapy.
Patients who are able to proceed through these desensitisation protocols can often tolerate considerable quantities of the food while on therapy, which would provide significant protection against accidental ingestions. The majority of patients experience some side effects of therapy, especially oropharyngeal symptoms and abdominal pain, but more severe reactions, including anaphylaxis, can occur. Side effects, especially gastrointestinal effects, often limit a patient’s ability to achieve the target maintenance dose. Studies have reported that ≤30% of patients fail to complete desensitisation,16 and rates of sustained unresponsiveness have been low.17,18 The optimal duration of immunotherapy is unknown, but it is likely that treatment of a long duration promotes sustained unresponsiveness.17 A Phase III trial investigating an experimental OIT for peanut allergy (AR101) was recently published at the end of 2018.19 Participants aged 4–55 years old who completed the regimen (i.e., received 300 mg per day of the maintenance regimen for approximately 24 weeks) underwent a double-blind, placebo-controlled food challenge (DBPCFC) at trial exit. In the study, 551 participants received AR101 or placebo, of whom 496 were 4–17 years old. Of the 372 participants who received active treatment, 250 (67.2%) were able to ingest a dose of ≥600 mg peanut protein without dose-limiting symptoms at the exit food challenge, compared with 5/124 participants (4.0%) who received placebo (difference= +63.0%; 95% confidence interval [CI]: 53.0–73.3; p<0.001). Efficacy was not shown in participants aged ≥18 years. Treatment with AR101 resulted in higher doses of peanut protein that could be ingested without dose-limiting symptoms and lower symptom severity during peanut exposure at the exit food challenge compared with placebo.
OMALIZUMAB
The U.S. Food and Drug Administration (FDA) approved OMB (Xolair®), a humanised anti-IgE mouse monoclonal antibody, for treating mild-to-severe allergic asthma and chronic spontaneous urticaria.20-23 OMB acts by binding to circulating free IgE; therefore, OMB reduces the amount that would normally be available to bind FcεRI on mast cells and basophils. In an early Phase I study of 15 allergic and asthmatic patients with serum levels of IgE between 187 and 1,210 ng/mL, intravenous injection of OMB resulted in a reduction of IgE to 1% of the pretreatment levels.24 Binding of IgE to FcεRI on mast cells and basophils enhances FcεRI expression;25-29 consequently, a reduction in free IgE by OMB leads to diminished FcεRI expression on the surface of mast cells, basophils, and dendritic cells.24,29-31 In a previous study, treatment of atopic individuals with OMB for 3 months reduced FcεRI expression in basophils by 97% from 220,000 to 8,300 receptors per basophil.24 An in vitro study with in situ-matured mast cells from human skin showed that IgE-dependent enhancement of FcεRI on human skin mast cells was prevented and reversed by OMB.29 In this study, OMB prevented upregulation of FcεRI by 90% when added simultaneously with polyclonal IgE at a molar ratio of 2.9 (OMB to IgE). Additionally, OMB dose-dependently decreased FcεRI expression in human skin mast cells when added to cultures after FcεRI had already been upregulated with IgE, which suggested that OMB could disassemble preformed IgE–FcεRI complexes; this was later confirmed with a cell-free system and human basophils.32,33 The efficacy and safety of OMB as a treatment against allergic asthma and urticaria have clearly been demonstrated, including as an add-on therapy with traditional treatments, such as glucocorticoids.20,21 The therapeutic potential of OMB in other IgE-mediated disorders in which FcεRI plays a role, including food allergies,34-36 allergic rhinitis,37,38 and atopic dermatitis,39,40 has also been shown. However, OMB is not available for children with severe bronchial asthma with >1,500 IU/mL of total IgE, those <6 years old, and those with severe food allergies. The effect of OMB in bronchial asthma was reported to be related to free IgE levels.41 In patients with high IgE levels (>1,500 IU/mL), high-volume OMB administration is required to maintain low free IgE concentrations (≤10 ng/mL). The maximum doses administered are limited by the product of IgE levels, body weight, and age. This explains why OMB is not available for patients with high IgE levels.
OMALIZUMAB COMBINED WITH ORAL IMMUNOTHERAPY FOR FOOD ALLERGIES
Milk Allergies
In 2011, OMB–OIT was administered to 13 patients with severe cow’s milk (CM) allergy in the USA.42 The OMB–OIT combination was efficacious in 11/13 patients; this finding suggests that OIT can be escalated more rapidly when combined with OMB, although adverse reactions are still relatively common.
Wood et al.43 studied the addition of OMB or placebo to open-label milk OIT. Open-label milk OIT was initiated after 4 months of OMB/placebo with escalation to maintenance over 22–40 weeks, followed by daily maintenance dosing to 28 months. At Month 28, OMB was discontinued, and subjects who passed an oral food challenge (OFC) continued OIT for 8 weeks. After this time, OIT was discontinued with a rechallenge at Month 32 to assess sustained unresponsiveness (SU), which was defined as the ability, after several months of OIT and subsequent avoidance of consuming the offending food for 4–8 weeks, to consume 2–4 g of the offending food allergen without developing clinically significant symptoms.44 At Month 28, 24 (88.9%) OMB-treated subjects and 20 (71.4%) placebo-treated subjects passed the 10 g ‘desensitisation’ OFC (p=0.18). At Month 32, SU was achieved by 48.1% of subjects in the OMB group and 35.7% of subjects in the placebo group (p=0.42). Adverse reactions were markedly reduced during OIT escalation in OMB-treated subjects for percentages of doses per subject provoking symptoms (2.1% versus 16.1%; p=0.0005), dose-related reactions requiring treatment (0.0% versus 3.8%; p=0.0008), and doses required to achieve maintenance (198 versus 225; p=0.008). The study by Wood et al.43 reported significant improvements in measurements of safety, but not in outcomes of efficacy (desensitisation and SU).
A pilot study with OIT in combination with OMB was planned, which has been accepted as a treatment for severe asthma, and reported successful desensitisation in a boy with severe CM allergy.45 On the basis of these observations, a pilot study to evaluate the efficacy and safety of OIT combined with 24 weeks of OMB for inducing desensitisation in children with a CM allergy compared with an untreated group was conducted.46 This study was a prospective, randomised, controlled trial in which 16 patients (aged 6–14 years) with high IgE levels and CM were enrolled. Patients were randomised 1:1 to receive OMB–OIT (treated group) or they were untreated (untreated group). The primary outcome was induction of desensitisation at 8 weeks after OMB was discontinued in the treated group and at 32 weeks after study entry. None of the 6 children in the untreated group developed desensitisation to CM, but all of the 10 children in the treated group achieved desensitisation (p<0.001). A significantly decreased wheal diameter in response to a skin prick test using CM was found in the treated group (p<0.050). These data suggest that OIT combined with OMB using microwave-heated CM may help to induce desensitisation for children with a high-risk CM allergy. The results of this randomised trial suggest that patients with high specific milk IgE levels are more likely to develop allergic symptoms after stopping OMB than those whose IgE levels are not high. Future studies regarding the therapeutic duration and dosages of OMB administration are required.
Non-milk Allergies
A pilot trial of peanut OMB–OIT was reported in 2013 and its efficacy was reported in 12/13 patients.47 In a recent study, MacGinnitie et al.48 reported a randomised, controlled trial on OMB–OIT with peanuts. In the study, 37 subjects were randomised to OMB (n=29) or placebo (n=8). After 12 weeks of treatment, subjects underwent a rapid 1-day desensitisation of ≤250 mg of peanut protein, followed by weekly increases of ≤2,000 mg. OMB was then discontinued and subjects continued on 2,000 mg of peanut protein. The subjects underwent an open challenge of 4,000 mg of peanut protein 12 weeks after stopping the study drug. If tolerated, subjects continued on 4,000 mg of peanut protein daily. The median peanut dose that was tolerated on the initial desensitisation day was 250.0 mg for OMB-treated subjects versus 22.5 mg for placebo-treated subjects. Subsequently, 23/29 (79.0%) subjects who were randomised to OMB tolerated 2,000 mg of peanut protein 6 weeks after stopping OMB versus 1/8 (12.5%) subjects who received placebo (p<0.01). Furthermore, 23 subjects who received OMB versus 1 subject who received placebo passed the 4,000 mg food challenge. Overall reaction rates were not significantly lower in OMB-treated versus placebo-treated subjects (odds ratio: 0.57; p=0.15), although OMB-treated subjects were exposed to much higher peanut doses. OMB allows subjects with a peanut allergy to be rapidly desensitised over as little as 8 weeks of peanut OIT. In the majority of subjects, this desensitisation is sustained after OMB is discontinued.
Andorf et al.36 reported anti-IgE treatment with OIT in multifood-allergic participants in a double-blind, randomised, controlled trial at the end of 2017. Enrolled in the study were participants who were aged 4–15 years with multifood allergies and validated by double-blind, placebo-controlled food challenges to their offending foods. Inclusion criteria included a positive skin prick test of ≥6 mm (wheal diameter, > the negative control), a food-specific serum IgE level >4 kU/L for each food, or both, and a positive DBPCFC at ≤500 mg of food protein. Exclusion criteria included eosinophilic oesophagitis and severe asthma. Participants were randomised 3:1 to receive multifood OIT for 2-5 foods, together with OMB (n=36) or placebo (n=12). Additionally, 12 individuals who fulfilled the same inclusion and exclusion criteria were included as controls. These individuals were not randomised and received neither OMB nor OIT. OMB or placebo was administered subcutaneously for 16 weeks once every 2 or 4 weeks and the doses administered were defined according to the manufacturer’s instruction. OIT started at Week 8 and continued before the DBPCFC at Week 36. On the initial dose-escalation day, patients received an initial dose of 5 mg food protein (divided equally among the number of foods included), with increasing doses administered every 30 minutes until reaching 1,250 mg or a maximum-tolerated dose. The participants then continued self-administration of the combined OIT at the maximum-tolerated dose at home, returning every 2–4 weeks for an increase in their daily dose (build-up phase). When participants reached the maintenance dose of 2 g per food, this dose was maintained daily (maintenance phase) until the food challenge at Week 36. The primary endpoint was the proportion of participants who passed a double-blind, placebo-controlled food challenge to 2 g protein from ≥2 of their offending foods. A total of 165 participants were assessed for eligibility of whom 84 did not meet the inclusion criteria and 21 declined to participate. The authors enrolled and randomised 48 eligible participants and the remaining 12 patients were included as nonrandomised, untreated controls. At Week 36, a significantly greater proportion of participants in the OMB group (30/36 [83%]) than those in the placebo group (4/12 [33%]) passed DBPFC (odds ratio: 10.0; 95% CI: 1.8–58.3; p=0.0044). All participants completed the study and there were no serious or severe (Grade 3 or worse) adverse events. Participants in the OMB group had a significantly lower median per-participant percentage of oral immunotherapy doses associated with any adverse events compared with the placebo group (27% versus 68%; p=0.0082). The most common adverse events in both groups were gastrointestinal events. In multifood-allergic patients, OMB enabled safe and rapid desensitisation. The above-described clinical trials are shown in Table 1. Randomised clinical trials and blinded trials were performed in 4/5 trials and 3/5, respectively; from these, 2/5 trials evaluated SU. In multifood and CM-allergic patients, OMB enabled safe and rapid desensitisation; whereas in multifood patients, OMB was efficacious, but not in CM patients. In the trial, OMB combined with OIT in patients with a CM allergy was efficacious in CM desensitisation compared with untreated patients with a CM allergy. Taken together, these five trials suggest that OMB is efficacious for desensitisation without severe adverse symptoms during OMB administration.
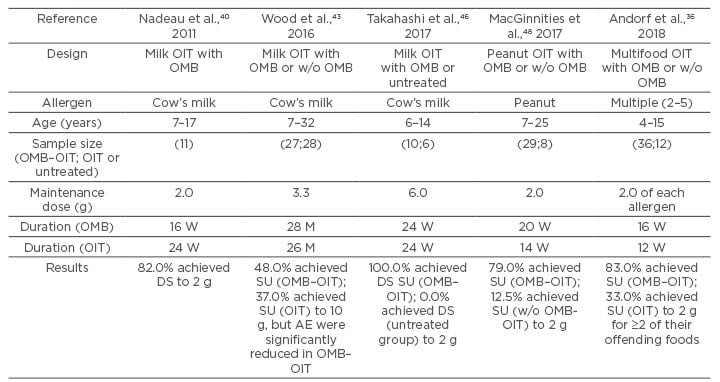
Table 1: Summaries of clinical trials.
DS: desensitisation; M: months; OIT: oral immunotherapy; OMB: omalizumab; OMB–OIT: oral immunotherapy combination with omalizumab; SU: sustained unresponsiveness; W: weeks; w/o: without.
IMMUNOTHERAPIES WITHOUT ORAL IMMUNOTHERAPY FOR FOOD ALLERGIES
SLIT is generally used for allergic rhinitis provoked by an environmental allergen. SLIT for treating food allergies has largely concentrated on peanuts, whereas other foods studied include milk and hazelnuts, as well as peaches and kiwifruit. A double-blind, placebo-controlled study assessed peanut SLIT compared with placebo for 12 months in 18 children.49 The treatment group allowed intake of 20 times more peanut protein than in the placebo group, with a median dose of 1,710 mg compared with 85 mg (p=0.011). SLIT appeared to be safe and relatively well tolerated, and its main side effects were largely oropharyngeal. A randomised, double-blind, placebo-controlled study evaluated 40 patients and compared peanut SLIT with placebo.50 The initial active SLIT subjects were treated to Week 44 with ≤1,386 μg of peanut protein SLIT daily.
At Week 44, peanut SLIT and placebo subjects completed a 5 g OFC and were unblinded, while placebo crossover subjects after unblinding at Week 44 were escalated to a higher dose peanut SLIT ≤3,696 μg daily (designated the high dose crossover group; the original peanut SLIT group maintained a maximum dose of 1,386 μg of peanut protein). After 44 weeks, 14/20 patients who received active treatment were considered responders. Of these patients, 3/20 patients who received placebo were considered responders. In the active treatment group, the median consumed dose increased from 3.5 to 496.0 mg at 44 weeks and this increased further to 996.0 mg at 65 weeks. Dose-related symptoms were reported for 18.3% of doses in the high-dose crossover subjects following 44 weeks of active therapy and for 18.1% doses received by peanut SLIT subjects following 44 weeks of active therapy. No subjects had severe dosing related symptoms and no dosing related reaction required treatment with epinephrine. A 3-year follow-up showed that 50% of patients had discontinued therapy51 and 4/37 (10.8%) patients were desensitised to 10 g of peanut powder and achieved SU as measured by an OFC after 8 weeks off SLIT. Thus, peanut SLIT induced a modest level of desensitisation and had an excellent long-term safety profile. However, most patients discontinued therapy by the end of Year 3, and only 10.8% of subjects achieved sustained unresponsiveness. The reasons for discontinuation after 3 years might be explained by the difficulty of maintaining daily therapies, mild oral discomfort (17.8% of doses), and a lack of robust responses as measured during OFC.
Two randomised studies have compared SLIT with OIT. One double-blinded, placebo-controlled trial evaluated peanut SLIT compared with peanut OIT.52 The SLIT maintenance dose was 3.7 mg and the OIT maintenance dose was 2,000 mg during a 12-month trial. The OIT group showed a much greater scale of change (141-fold increase) compared with the SLIT group (22-fold increase). The OIT group was more likely to have more severe reactions than the SLIT group.
Another study assessed milk SLIT with milk SLIT followed by OIT with 60 weeks of maintenance therapy in 30 patients.53 In the study, 14/20 patients who received OIT passed an OFC with 8 g of milk compared with 1/10 patients who received SLIT (p=0.002). Patients who received OIT were more likely to have systemic adverse events compared with patients who received SLIT.
EPIT delivers even smaller dosages of the antigen than does SLIT. EPIT appears to be a relatively safe form of immunotherapy. A recent Phase III, multicentre, randomised, double-blind, placebo-controlled trial54 conducted EPIT for the treatment of peanut allergy. Participants included peanut-allergic children (aged 4–11 years [n=356] without a history of severe anaphylactic reaction) developing objective symptoms during a DBPCFC at an eliciting dose of ≤300 mg peanut protein. Daily treatment was with a peanut patch containing 250 μg peanut protein (n=238) or placebo (n=118) for 12 months. In this randomised clinical trial of 356 peanut-allergic children, differences in the treatment response rate (percentage of participants meeting a defined eliciting dose to peanut challenge) after 12 months of treatment with peanut-patch therapy was statistically significant compared with placebo (35.3% versus 13.6%), but did not meet a prespecified criterion (15.0% lower bound of the CI) for a positive trial result. The EPIT study reported a statistically significant response in peanut-allergic children compared with placebo, but the study did not meet a component of the primary outcome.
CURRENT STATUS AND FUTURE PROSPECTS
The efficacy and risk of each immunotherapy is shown in Figure 1. OIT has shown the greatest promise for efficacy in terms of the amount of protein that can be ingested. However, OIT has less tolerability and a less favourable safety profile compared with SLIT and EPIT. EPIT offers the least protection but has the best safety and tolerability profile. Investigation is currently underway for modified antigens that may be used for immunotherapy and for adjuncts that may help facilitate immunotherapy, including biologics such as anti-IgE therapy. The combination of OIT with OMB has extremely high medical costs. SLIT and EPIT are extremely safe and highly effective, but there have only been a small number of clinical trials, and thus their effectiveness is controversial. Additionally, only two modalities (AR101 from Aimmune Therapeutics, California, USA,19 and Viaskin Peanut from DBV Technologies, France)54 have completed fully powered Phase III studies and only AR101 is being reviewed by regulatory authorities at this time.
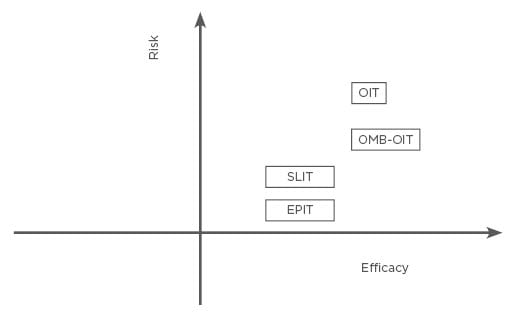
Figure 1: The efficacy and risk of oral immunotherapy, sublingual immunotherapy, epicutaneous immunotherapy, and oral immunotherapy combination with omalizumab.
EPIT: epicutaneous immunotherapy; OIT: oral immunotherapy; OMB: omalizumab; OMB–OIT: oral immunotherapy combination with omalizumab; SLIT: sublingual immunotherapy.





