Abstract
Allergic conjunctivitis is predominantly an immunoglobulin E-mediated hypersensitivity reaction to environmental allergens. Allergic diseases affect >30% of the world’s population, of which 40% report associated ocular manifestations. Cellular and soluble mediators play a major role in the pathophysiology of allergic conjunctivitis. Mast cells, which are major effector cells of allergic conjunctivitis, undergo activation and degranulation to release histamine, tryptase, prostaglandins, leukotrienes, and cytokines. These mediators play important roles in immunopathological mechanisms that generate the clinical manifestations of allergic conjunctivitis. These clinical features include conjunctival hyperaemia, chemosis, tearing, itching, papillae, mucus discharge, and eyelid oedema. Histamine mediates the early phase of the allergic immune response, whereas lipid mediators and cytokines are involved in the late phase of the immunopathology of allergic conjunctivitis. Current management of allergic conjunctivitis includes non-pharmacological approaches such as allergen avoidance and palliative therapy, whereas pharmacological therapeutic modalities may include antihistamine–mast cell stabiliser combination ophthalmic formulations and allergen-specific immunotherapy. Furthermore, as cellular and soluble mediators play a pivotal role in the immunopathogenesis and immunopathology of allergic conjunctivitis, development of immunotherapeutic and pharmacotherapeutic agents specific to these mediators can enhance the therapeutic index and safety profile of anti-allergy treatment.
INTRODUCTION
Allergic hypersensitivity reaction affecting the ocular surface is either immunoglobulin (Ig)E and/or T cell mediated, with allergic conjunctivitis being predominantly an IgE-mediated hypersensitivity immune reaction to environmental allergens.1 The two forms of allergic conjunctivitis are seasonal allergic conjunctivitis and perennial allergic conjunctivitis. Seasonal allergic conjunctivitis is due to outdoor airborne allergens. The common aeroallergens are tree and grass pollen, which are common during the spring and summer months. Perennial allergic conjunctivitis is a year-round variant of allergic conjunctivitis, which is associated with dust mites, mould, and pet dander.2,3 Allergic disease affects more than a third of the world’s population and >40% of these individuals have ocular involvement associated with their allergic condition.4 The quality of life of individuals with allergic conjunctivitis is affected, with a significant impact on work productivity and school performance.5 This review focusses on the immunopathogenesis, immunopathology, clinical manifestations, and immunotherapeutic modalities of allergic conjunctivitis.
CONJUNCTIVA: AN IMMUNOLOGIC ACTIVE BARRIER
Conjunctiva is an immunologically active mucous membrane that consists of epithelial and subepithelial layers.6,7 The conjunctival subepithelial layer is a fibrovascular connective tissue consisting of blood vessels, immune cells, and non-immune cells. Goblet cells, intraepithelial lymphocytes, and Langerhans cells are found in the conjunctival epithelial layer.7,8 Macrophages and connective tissue-type mast cells are found only in the subepithelial layer (conjunctival stroma) of the conjunctiva, whereas eosinophils are not present in the normal conjunctiva.7,9 The distribution of immune cells in the conjunctiva constitutes the mucosal immune system of the conjunctiva known as the conjunctiva-associated lymphoid tissue (CALT).10 It is biased toward inducing anti-inflammatory immunity that favours the generation of IgA-producing plasma cells, regulatory T cells, and immunosuppressive cytokines (e.g., interleukin 10), which provide immunological protection for the cornea. Diffuse lymphoid effector tissue is the efferent arm of CALT and consists mainly of effector lymphocytes. Follicle-organised inductive lymphoid tissue is the afferent arm of CALT and consists of B cells, T cells, and apical follicle-associated epithelium with microfold cells. It serves as a site of localised immune processing of antigens that pass through the conjunctival epithelium.11
Mast cells are major effector cells of allergic inflammation of the conjunctiva, of which the connective tissue type that contains tryptase and chymase are found in the conjunctiva stroma.1,12 Histamine is a low molecular weight biogenic amine secreted by degranulated conjunctival mast cells and plays an important role in the immune and pathological mechanisms of allergic conjunctivitis via interaction with histamine receptors. It is noteworthy that histamine receptors are members of the G protein-coupled receptors.3,13,14 Histamine is produced by L-histidine decarboxylate-mediated decarboxylation of histidine and it is degraded by histamine N-methyl transferase and diamine oxidase.14-17 Histamine 1 receptor (H1R) and histamine 2 receptor are expressed on nerve cells, vascular smooth muscles, endothelial cells, epithelial cells, neutrophils, eosinophils, monocytes, macrophages, dendritic cells (DC), goblet cells, T cells, and B cells.18,19 Histamine 3 receptors are expressed on nerve cells and goblet cells.8 Histamine 4 receptors are expressed on mast cells, goblet cells, basophils, eosinophils, monocytes, macrophages, T cells, basophils, and DC.17,19 The healthy conjunctiva epithelium has intercellular junctions that help maintain its barrier function of preventing allergens from gaining access to the subepithelial layer;20 however, in allergic conjunctivitis, disruption of the conjunctival epithelial barrier function through the activation of the protease-activating receptor is likely to play a role in the pathogenesis of allergic conjunctivitis.21 Furthermore, epithelial cells have an active role in allergen-induced conjunctival inflammation through the action of cytokines, chemokines, thymic stromal lymphopoietin (TSLP), and adhesion molecules they release during the allergic response. These mediators promote the influx of immune cells into the site of allergic inflammation.22-24
Toll-like receptors (TLR) are pattern recognition receptors expressed on both non-immune (epithelial, fibroblast) and immune cells (macrophages, DC, lymphocytes, mast cells) of the conjunctiva.25,26 Interaction between an appropriate TLR ligand and a TLR initiates a signalling cascade that leads to the generation of cytokines.8 Activation of TLR-2 and TLR-4 expressed on mast cells is associated with degranulation of activated mast cells and release of cytokines, and, as such, it is most likely that increased expression of TLR on the conjunctiva occurs during ocular allergic conditions.26 The conjunctiva expresses transient receptor potential (TRP) channels, such as TRPV1, found on sensory nerve fibres, epithelial cells, and mast cells.27 Activation of TRPV1 on conjunctival epithelial cells and mast cells is associated with ocular itch.28
Fibroblasts are cells that produce compounds that make up the extracellular matrix and provide structural support to the conjunctiva.29 Additionally, conjunctival fibroblasts can act as immune modulators in allergic conjunctivitis by expressing chemokines (e.g., thymus and activation- regulated chemokine or chemokine [C-C motif] ligand 17 [CCL17]), matrix metalloproteinase, and adhesion molecules (e.g., intercellular adhesion molecule 1) in response to stimulation by IL-4 released by degranulated mast cells. It is noteworthy that thymus and activation-regulated chemokines are potent chemoattractants for Type 2 helper (Th2) cells.30,31
IMMUNOPATHOGENESIS: THE SENSITISATION PHASE OF ALLERGIC CONJUNCTIVITIS
Exposure of the conjunctiva to allergens initiates the immunopathogenesis of allergic conjunctivitis, which is initially characterised by the sensitisation of conjunctival mast cells by allergen-specific IgE. Protease produced by environmental allergens activate protease-activated receptor-2 in the conjunctiva, culminating in a breakdown of the tight junction between conjunctival epithelial cells and subsequent entry of the allergen into the subepithelial layer, where they have access to immune cells.32 Conjunctival DC engulf and process these allergens, changing them into peptides that are complexed to major histocompatibility complex Class II molecules on the mature DC. Additionally, to ensure activation of naïve T cells in the regional secondary lymphoid organs, there is upregulation of CD80 and CD86, costimulatory molecules required for providing the costimulatory signal during the activation of naïve T cells. In the regional lymph node, the interaction of peptide–major histocompatibility complex Class II on the mature DC with T cell receptor on naïve CD4 T cells results in activation, proliferation, and differentiation of CD4 T cells into allergen-specific Th2 cells. Allergen-specific Th2 cells release IL-4, a cytokine that induces the proliferation and differentiation of allergen-specific B cells into plasma cells that produce allergen-specific IgE. The interaction between CD40 on allergen-specific B cell and CD40 ligand on allergen-specific Th2 cell is associated with allergen-specific Th2 cells releasing IL-4, which induces the proliferation and differentiation of allergen-specific B cells into plasma cells that produce allergen-specific IgE.33 Conjunctival mast cells become primed when allergen-specific IgE binds via its Fc region to the Ig-like domain of the alpha chain of Fc Epsilon Receptor I (FcεRI) on the surface of conjunctival mast cells.34,35
Environmental allergens possess proteolytic capabilities that cause damage to the epithelium; subsequently, damaged epithelial cells release pro-allergic cytokines, such as IL-33.36 Additionally, TSLP is a pro-allergic cytokine released by allergen-activated conjunctival epithelium. Li et al.37 demonstrated that short ragweed pollen, an agonist for TLR4, is capable of inducing conjunctival epithelial cells to produce TSLP via the TLR4-dependent innate immunity pathway. TSLP interact with TSLP receptors on conjunctival DC, leading to upregulation of OX40 ligand expression. TSLP-activated conjunctival DC expressing OX40 ligand migrates to the regional lymph node to promote Th2 differentiation via OX40 ligand and OX40 interactions.38,39 It is noteworthy that TSLP inhibit IL-12p40 expression in DC, allowing for conjunctival DC to activate naïve CD4 T cells to differentiate into Th2 cells.40 Furthermore, Li et al.41 demonstrated that pollen interacts with TLR4 on ocular surface epithelium in innate immunity to induce the conjunctival epithelium to secrete IL-33, a pro-allergic epithelial cytokine that promotes Th2 cell differentiation. Thus, Th2 cells and conjunctival epithelial cells participate in initiating priming of conjunctival mast cells in the sensitisation phase of allergic conjunctivitis (Figure 1).1,33,39 Innate lymphoid cells (ILC), grouped into three cell types on the basis of cytokine and transcription factor profile, may have a role in allergic eye disease. ILC2 has similar lymphoid morphology and phenotype to Th2 cells, but lacks an antigen receptor. Epithelial cell and myeloid cell-derived IL-25, IL-33, and TSLP can activate and recruit ILC2, which, in turn, releases IL-4 that plays a role in Th2 cell differentiation.36 Innate T cells, such as γδT cells and natural killer T cells, have been shown to maximise the expression of allergic conjunctivitis. Both natural killer T cells and γδT cells can secrete IL-4, which promotes the differentiation of CD4 T cells into allergen-specific Th2 cells.42,43 Furthermore, though Th2 cells are the predominant mediators in ocular allergy, evidence exists of other Th cell subsets participating in allergic eye disease. Th1, Th2, Th17, and regulatory T cells are other subsets of CD4 T helper cells, of which Th1 and Th17 cells, in addition to Th2 cells, play a role in severe chronic forms of ocular allergy, such as atopic keratoconjunctivitis (AKC) and vernal keratoconjunctivitis (VKC).40
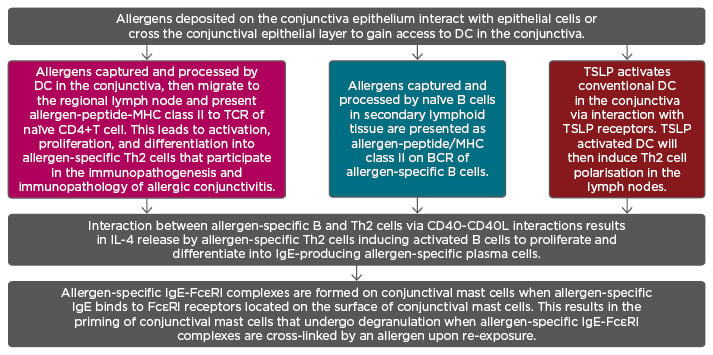
Figure 1: Sensitisation phase of allergic immune response is associated with generation of allergen-specific immunoglobulin E antibodies that bind to FcεRI receptors on the surfaces of conjunctival mast cells.
Note: The three colours depicted represent three routes of Th2 polarisation.
BCR: B cell receptor; DC: dendritic cells; IgE: immunoglobulin E; IL-4: interleukin 4; MHC: major histocompatibility complex; TCR: T cell receptor; Th2: Type 2 helper; TSLP: thymic stromal lymphopoietin.
IMMUNOPATHOLOGICAL MECHANISMS: THE EARLY PHASE OF ALLERGIC CONJUNCTIVITIS
Re-exposure of the conjunctiva to allergens that induced the priming of conjunctival mast cells leads to early and late phase immunopathological responses characteristic of allergic conjunctivitis. The early phase allergic response is initiated by the binding of multivalent allergens to the IgE–FcεRI complex on primed conjunctival mast cells. This is followed by crosslinking of IgE–FcεRI complexes on primed conjunctival mast cells causing activation and subsequent degranulation of primed conjunctival mast cells.4,33,44,45 Degranulation of mast cells results in immunobiological reactions that occur in response to the release of preformed mediators, such as biogenic amines (e.g., histamine) and neutral proteases (e.g., tryptase). Release of preformed mediators is followed by synthesis and release of lipid mediators (e.g., prostaglandins, leukotrienes) and cytokines (e.g., IL-4).4,33 This outlines the activation phase of allergic response in allergic conjunctivitis. The signs and symptoms of the early phase of allergic conjunctivitis include hyperaemia, chemosis, tearing, itching, and eyelid oedema. The clinical manifestations of the early phase allergic reaction are attributed to the action of histamine on the vascular endothelium of conjunctival blood vessels, conjunctival sensory nerve fibres, immune cells, and resident cells in the conjunctiva and conjunctival epithelium. When histamine interacts with H1R and histamine 2 receptor on conjunctival blood vessels, it induces conjunctival vasodilation and increased capillary leakage, which cause conjunctival hyperaemia and chemosis, respectively. Interaction between histamine and H1R on conjunctival sensory nerve fibres is associated with the patient’s complaint of ocular itch. Histamine interacts with histamine receptors on DC leading to maturation of dendritic cells. Histamine-activated DC are capable of activating naïve CD4 T cells to differentiate into allergen-specific Th2 cells that secrete IL-4, a cytokine that activates conjunctival fibroblasts to undergo proliferation and increased collagen synthesis, which manifests as papillae on the palpebral conjunctiva (Figure 2). Additionally, Ahadome et al.46 demonstrated that conjunctival conventional DC-derived aldehyde dehydrogenase mediates the inflammation-induced conjunctival fibroproliferative pathological changes in a mouse model of severe allergic disease via its synthesis of retinoic acid, following interaction between DC and conjunctival fibroblasts.
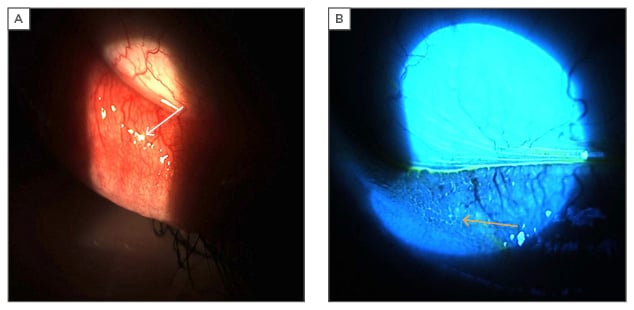
Figure 2: Photographs of inferior palpebral conjunctiva papillae in a patient with seasonal allergic conjunctivitis.
A: white light on inferior palpebral conjunctiva with reflections off of papillae (white arrow); B: blue light on inferior palpebral conjunctiva with fluorescein dye pooling between papillae (yellow arrow).
Furthermore, the binding of histamine with its receptors on the conjunctival epithelium is associated with breach of the barrier function of the conjunctival epithelium and expression of adhesion molecules, chemokines, and cytokines from histamine-activated conjunctival epithelial cells.4,8,15,16,33,45,47 Interaction between histamine and H4R on mast cells and Th2 cells is associated with recruitment of these cells to the site of allergen-induced conjunctival inflammation to potentiate the histamine-induced allergic inflammation with remodelling of the conjunctiva.19,48 In addition to the role of IL-4 in remodelling the palpebral conjunctiva, tryptase released along with histamine during the early phase is known to trigger the proliferation of conjunctival fibroblasts, which results in papillary hypertrophy of the palpebral conjunctiva. It is notable that histamine and tryptase are biomarkers of IgE-mediated mast cell activation and degranulation in the early phase of allergic conjunctivitis.4 Thus, conjunctival fibroblasts promote allergen-induced conjunctival inflammation and tissue remodelling in allergic conjunctivitis.49 It is also noteworthy that patients with AKC and VKC present with significant papillary hypertrophy of the lower and superior palpebral conjunctiva, respectively. Furthermore, limbal gelatinous hyperplasia is another conjunctival fibroproliferative lesion in VKC and AKC.1,50
IMMUNOPATHOLOGICAL MECHANISMS: THE LATE PHASE OF ALLERGIC CONJUNCTIVITIS
Additionally, degranulated mast cells release lipid mediators, such as prostaglandins and leukotrienes, which play a role in the early stages of the late phase allergic response and potentiate the histamine-mediated clinical expression of allergic conjunctivitis. Prostaglandin causes conjunctival hyperaemia by inducing vasodilation of conjunctival blood vessels. It also intensifies the histamine-mediated ocular pruritus. Leukotrienes induce vasodilation and increased permeability of conjunctival blood vessels, which manifest as conjunctival hyperaemia and chemosis, respectively.33 Additionally, prostaglandin D2 and leukotriene D4 may induce the recruitment and activation of ILC2.36 Cytokines (e.g., IL-4, IL-5, and IL-13) and chemokines (e.g., CCL17) are released from degranulated conjunctival mast cells. Cytokines play a role in activating conjunctival epithelial cells and fibroblasts, as well as recruiting and activating eosinophils in the late phase. These cytokine-activated conjunctival cells release chemokines and adhesion molecules that recruit inflammatory cells to the site of allergic inflammation. IL-5 recruits and activates eosinophils, a type of immune cell that participates in the late phase of allergic conjunctivitis, where it plays a role in tissue damage and ocular surface remodelling, particularly in perennial allergic conjunctivitis and chronic forms of ocular allergy. Intercellular adhesion molecule 1 plays a role in the recruitment of immune cells, including lymphocytes into the conjunctiva, which leads to the persistence and exacerbation of chronic allergen-induced conjunctival inflammation.2,33,50 IL-4 and IL-13 stimulate conjunctival fibroblasts to produce vascular endothelial growth factor, which plays a role in remodelling the palpebral conjunctiva, resulting in papillary hypertrophy of the palpebral conjunctiva.51 As such, conjunctival mast cell-derived cytokines and chemokines in conjunction with prostaglandins and leukotrienes contribute to the immunopathology of the late phase of allergic conjunctival inflammatory reaction.1
Conjunctival hyperaemia and chemosis are ocular findings attributed to the action of histamine and leukotrienes (Figure 3).2,4,33 The hallmark symptom of ocular itch is mediated by the interaction between histamine and its receptor on conjunctival sensory fibres. TRPV1 activation by leukotriene B4 is associated with ocular itching via interaction with LTB4 and its receptors on sensory nerve fibres; histamine-induced itching requires the activation of TRPV1 on sensory nerve fibers.52 Mucoid secretion in allergic conjunctivitis is associated with oversecretion of mucus by histamine-activated goblet cells in the conjunctiva8 and leukotriene (C4 and D4)-activated goblet cells.2,4,33 Conjunctival fibroproliferative lesions in allergic conjunctivitis are due to infiltration of the conjunctiva with immune cells recruited by chemokines and adhesion molecules,1,2,33,50 as well as the action of conjunctival DC-derived aldehyde dehydrogenase.46
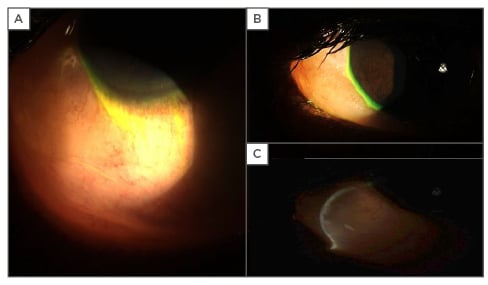
Figure 3: Photographs of severe bulbar conjunctival chemosis secondary to allergy.
A and B: severe conjunctival chemosis of temporal conjunctiva with marked elevation and billowing over lower eyelid and extending to corneal limbus; C: optic section through conjunctival chemosis indicates substantial underlying oedema causing striking elevation.
CURRENT AND EMERGING TREATMENTS IN OCULAR ALLERGY
Management of allergic conjunctivitis should include allergen avoidance, pharmacotherapy, immunotherapy, and patient education. Specifically, topical medications cover a wide range of pharmacological classes, including vasoconstrictors, antihistamines, mast cell stabilisers, dual action antihistamines/mast cell stabilisers, and corticosteroids. Although topical corticosteroids are highly effective in relieving the signs and symptoms of acute flare up of allergic conjunctivitis, their prolonged use can result in unwanted and serious sequelae. As such, the use of immunomodulators, such as calcineurin inhibitors, would provide comparable treatment efficacy without the side-effect profile associated with chronic corticosteroids in recalcitrant cases or chronic forms of ocular allergy, such as AKC and VKC. Cyclosporine is a calcineurin inhibitor that blocks the IL-2-induced proliferation of Th2 cells and inhibits histamine release from mast cells. Immunosuppressive effects of cyclosporine would be effective in controlling allergic conjunctival inflammation.50,53 Similarly, tacrolimus is a calcineurin inhibitor with a similar mechanism of action to cyclosporine but greater immunosuppressive potency. Attas-Fox et al.54 reported that tacrolimus 0.03% dermatologic ointment was safe and well-tolerated in providing therapeutic benefit for patients with intractable allergic conjunctivitis. In chronic forms of allergic conjunctivitis, both cyclosporine and tacrolimus, by virtue of their mechanism of action, can be helpful in intractable cases.
Another potential therapy for treating allergic rhinoconjunctivitis is omalizumab, a recombinant humanised monoclonal antibody that binds to the Fc portion of IgE to reduce the availability of free IgE for binding to FcεRI. Another mechanism of action of omalizumab is to dissociate IgE from the IgE–FcεRI complex on primed mast cells.55 Because allergic conjunctivitis is an IgE-mediated conjunctival inflammatory disease, there is a potential clinical use for omalizumab in the management of allergic conjunctivitis. Allergen-specific immunotherapy reduces the clinical expression and prevents recurrence of allergic diseases by inducing immunological tolerance to allergens. Allergen-specific immunotherapy for treating allergic conjunctivitis involves the administration of allergens into the conjunctiva, which then results in the induction of increased immune tolerance to the inciting allergen. This mode of allergen-specific immunotherapy would be beneficial for patients with perennial allergic conjunctivitis.56 Future consideration for targeted immunotherapy may include the blocking of TSLP signalling via a soluble TSLP receptor–Ig to decrease downstream signalling. This has been shown to be effective in pulmonary DC and may prove effective as an agent in attenuating TSLP-induced activation of DC that leads to the generation of allergen-specific Th2 cells.57 Schlereth et al.58 reported that blockade of C-C chemokine receptor Type 7 (CCR7) in immunised mice resulted in inhibition of allergic conjunctivitis. Because CCR7 is pivotal for chemokine-mediated trafficking of mature allergen-ladened DC to the lymph node, blockade of CCR7 may be therapeutically beneficial in individuals with allergic conjunctivitis. As such, most of the cells and mediators that participate in the immune and pathological mechanism of allergic conjunctivitis could be potential therapeutic targets for allergic conjunctivitis.
CONCLUSION
Allergic conjunctivitis is predominantly an IgE-mediated hypersensitivity reaction to environmental allergens. Cellular and soluble mediators play a major role in the immunopathogenesis and immunopathology of allergic conjunctivitis. Allergen-induced degranulated conjunctival mast cells release histamine, tryptase, prostaglandins, leukotrienes, and cytokines. Soluble mediators are also released by activated epithelial cells and fibroblasts in the conjunctiva. These mediators play important roles in immune and pathological mechanisms that generate the clinical manifestations of allergic conjunctivitis. Current management of allergic conjunctivitis includes non-pharmacological and pharmacological therapeutic modalities. Research into the emerging role of pattern recognition receptors in the immunopathogenesis of allergic conjunctivitis, as well as further studies on immune mechanisms of molecules derived from allergen-activated conjunctival epithelial cells and myeloid cells, would provide a clear understanding of their role in the immunopathogenesis and immunopathology of allergic conjunctivitis. Furthermore, it will be imperative for current and future research to broaden our understanding of the role of conjunctival macrophages in the immunopathogenesis and immunopathology of allergic conjunctivitis. Such knowledge is required for developing immunotherapeutic and pharmacotherapeutic agents with an enhanced safety profile and anti-allergic therapeutic index.






