Abstract
In the present systematic review and meta-analysis, the authors analysed case reports of drug-induced thrombocytopenia/drug-induced thrombocytopenic purpura (DITP) and its mechanisms. The search included electronic databases for case reports of DITP using specific keywords in MEDLINE via PubMed, PubMed Central, and Embase. All case reports were designated a score/criteria (definite, probable, or possible). The mechanism of DITP was also analysed in each case report. A total of 751 case reports were included in the meta-analysis. The incidences for all-score DITP by random and common effect models were 0.65% (95% confidence interval: 0.61–0.69) and, 0.65% (95% confidence interval: 0.62–0.68), respectively. The number of DITPs with scores of 1, 2, and 3 was found in 151, 300, and 300 patients, respectively. Amongst the drugs, the maximum number of DITPs were caused by antibiotics, antimalarials, monoclonal antibodies, antiplatelet drugs, disease-modifying antirheumatic drugs, anti-epileptics, anti-cancer chemotherapeutics, and non-steroidal anti-inflammatory drugs. Out of 751 cases, 478 patients were hospitalised, and 323 patients had external or internal bleeding, including 62 patients who had major bleeding intracranially or retroperitoneally and required transfusion of two or more units of red blood cells. Mortality occurred in 12 patients. Clinicians should be aware of the potential of drugs causing DITP as an important adverse event, as it may affect patient compliance and adherence to drugs. Unrecognised DITP may lead to severe thrombocytopenia and inappropriate patient management.
Key Points
1. Drug-induced thrombocytopenia/drug-induced thrombocytopenic purpura (DITP) is a potentially fatal adverse effect of drugs, which could be associated with external or internal bleeding varying from mild to severe. DITP affects patient compliance and adherence to drugs.
2. The present systematic review and meta-analysis analysed case reports of DITP. The drugs found to be commonly associated with DITP included antibiotics, monoclonal antibodies, antiplatelets, and disease-modifying antirheumatic drugs.
3. Early detection and clinicians’ awareness of DITP could prevent this adverse event, and result in the proper management of patients.
INTRODUCTION
Drug-induced thrombocytopenia/drug-induced thrombocytopenic purpura (DITP) is a potentially fatal side effect that is often under-recognised, and is characterised by immunogenic drug-dependent antibodies and non-immunogenic causing destruction of the platelets when the responsible drug is ingested or injected. Some of these antibodies are already present in a non-reactive form in the absence of a drug, but strongly bind to particular epitopes on platelet membrane glycoproteins (GP) IIb/IIIa or Ib/IX9 when the sensitising agent is available in soluble form. The mechanism could be platelet production inhibition, and/or favouring their elimination, or destruction from the peripheral blood. Cytotoxic drugs suppress overall haematopoiesis or affect megakaryocytopoiesis, which either causes increased platelet consumption/destruction or impaired platelet production.1 Numerous medications, including antimicrobials, non-steroidal anti-inflammatory drugs (NSAID), anticonvulsants, and sedatives have been linked to causing thrombocytopenia. Generally, low-molecular-weight drugs like penicillin and penicillin derivatives are considered more immunogenic, causing the production of drug (hapten)-specific antibodies, which are covalently attached to a carrier protein. These drugs in vivo may have been connected to certain membrane proteins, which were further identified as foreign particles by the immune system, resulting in the formation of drug-specific antibodies. On subsequent ingestion, the medication can reassociate with the membrane protein to produce an antibody target, leading to the destruction of the blood cells.
Skin bruises, petechiae, epistaxis, or more severe symptoms, including purpura, gum bleeding, gastrointestinal or urinary tract bleeding, and pulmonary haemorrhage can all be signs of DITP.2
Major bleeding is described as intracranial, retroperitoneal, or overt bleeding that requires the transfusion of at least two units of packed red blood cells, or results in an immediate haemoglobin drop of 2 g/dL.3 The lack of a standardised definition for thrombocytopenia, confirmatory testing, and voluntary reporting leads to under-report of cases of DITP. Clinically, it is challenging to differentiate between idiopathic thrombocytopenic purpura and other thrombocytopenia-causing conditions, such as concurrent sepsis or use of heparin products, which are further increased in patients with multiple comorbidities who are taking multiple drugs.4 Evidence is lacking with regard to the category of drugs and their association with DITP and its mechanism.
Hence, this systematic review and meta-analysis was undertaken with the objective to analyse case reports of the potential drugs, such as antibiotics, antimalarials, monoclonal antibodies, antiplatelets, disease-modifying anti-rheumatic drugs (DMARD), anti-epileptics, anti-cancer chemotherapeutics, NSAIDs, histamine-2 (H2)-blockers, anti-arrhythmics, antipsychotics, diuretics, anti-hypertensives, and vaccines associated with DITP. The authors also describe the mechanisms of DITP.
METHODS
Search Strategy
Electronic databases were searched, including MEDLINE via PubMed, Pubmed Central, and EMBASE, for case reports of DITP. The search process was conducted using the following keywords: “drug-induced purpura,” “drug-induced thrombocytopenia,” “drug-induced maculopapular rashes,” and “adverse-drug skin reaction, skin manifestations OR cutaneous manifestations OR urticaria OR skin disease OR skin lesions”. The references of relevant case reports were also reviewed for other potentially suitable studies. The preferred reporting items for systematic review and meta-analysis (PRISMA-2020) were followed for the study design (Figure 1).5
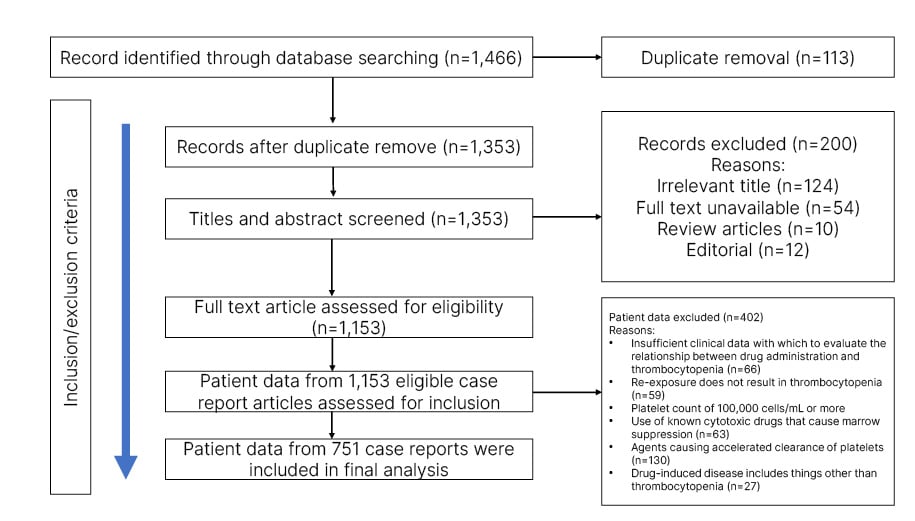
Figure 1: PRISMA diagram describing the case selection process.
Data Extraction
Literature retrieval, screening of the eligible studies, and data extraction of case reports were done by two authors independently, based on the title and abstract according to predefined criteria. Thereafter, they examined the full texts of the articles to achieve maximum reliability and preparation, co-interventions, and follow-up if available.
All case reports were assessed for patient characteristics, including age; sex; drug history; symptoms; bleeding definition; whether the patient required hospitalisation or not; and score, based on causality assessment as definite, probable, and/or possible. Any disagreements in selection or extraction were resolved by consensus.
Inclusion and Exclusion Criteria
The authors evaluated all case reports that were about DITP without any age or sex restriction. Studies reported sufficient data describing analysis, including confirmed drugs, bleeding or no bleeding, severity of bleeding, clinical symptoms, laboratory findings, outcomes, diagnostic methods, and treatment.
Inclusion criteria included: the candidate drug preceded thrombocytopenia and recovery from thrombocytopenia was complete and sustained after the drug was discontinued; re-exposure to the candidate drug resulted in recurrent thrombocytopenia; the candidate drug was the only drug used prior to the onset of thrombocytopenia, or other drugs were continued or reintroduced after discontinuation of the candidate drug with a sustained normal platelet count; and other aetiologies for thrombocytopenia were excluded.
Exclusion criteria included: case reports that did not provide clinical data to assess the association between medication administration and thrombocytopenia; case reports with platelet counts of 100,000 cells/mL or more; case reports with drugs having cytotoxic action, known to inhibit bone marrow and induce other abnormalities, such as aplastic anaemia, or thrombotic thrombocytopenic purpura-haemolytic uremic syndrome, and known to speed up the platelet clearance process; and cases in which a patient was exposed to non-therapeutic agents like environmental pollutants, illicit drugs, drug overdose, and drugs not currently in use.
CLASSIFICATION OF CASE REPORTS BASED ON THE MECHANISM OF DRUG-INDUCED THROMBOCYTOPENIA
Systematic Review
The authors systematically reviewed all published case reports of DITP and classified them into six groups on the basis of the mechanism of thrombocytopenia. Group 1 included drug-dependent antibodies; Group 2 autoantibody induction; Group 3 drug-specific antibodies; Group 4 fibrinogen receptor antagonist-dependent antibodies; Group 5 hapten-dependent antibodies; and Group 6 bone-marrow suppression and toxicity.
Criteria for article score: score 1–5 was given to each article as follows. Score 1: thrombocytopenia is definitely caused by a drug and inclusion criteria 1, 2, and 3 fulfilled; score 2: thrombocytopenia is likely brought on by medication, and inclusion criteria 1, 3, and 4 were fulfilled; score 3: thrombocytopenia possibly caused by medication, and inclusion criterion 1 was fulfilled; score 4: drug use is unlikely to induce thrombocytopenia; and score 5: when inclusion criterion 1 is not satisfied or pre-exposure does not induce thrombocytopenia.
A further three levels of association according to the following criteria were analysed in case reports. Firstly, ‘definite’ causal association of drug with thrombocytopenia, which required four criteria to be fulfilled: suspected drug preceded thrombocytopenia, and recovery from thrombocytopenia was complete and sustained after the drug was discontinued; the suspected drug was the only drug used prior to the onset of thrombocytopenia, or other drugs were continued or reintroduced with a sustained normal platelet count; other aetiologies for thrombocytopenia were reasonably excluded; and re-exposure to the suspected drug resulted in recurrent thrombocytopenia. Secondly, a ‘probable’ association required three criteria (1, 2, and more). Thirdly, a possible association if criterion 1 was met but criteria 2 and 3 were not met, the drug was interpreted as having a ‘possible’ association. The authors did not incoclude an analysis of lab reports for drug-dependent antibodies in this meta-analysis.
Meta-analysis
The data for each case report included in the analysis will be provided on demand. To estimate the proportion of reported cases of DITP, the authors performed a meta-analysis for the estimation of the summary effect. The R version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria) meta-package (metaprop and forest function) was used for data analysis. Drugs were classified based on the mechanism of DITP, and further models were selected based on heterogeneity among the mechanisms of DITP, as assessed using the Q statistic. P<0.10 was taken as the significance level. The I2 statistic was also calculated to measure heterogeneity. I2>50% was considered to be an indicator of high heterogeneity. Forest plots were drawn and presented individual mechanisms of DITP as horizontal solid lines with their confidence intervals (CI). Common effect and random effect models were applied to assess the incidence of DITP, and forest plots were plotted for the case reports categorised with scores 1–3 (definite, probable, and possible association) with their 95% CIs. The random-effects model of the average weightage of studies assumes that each study provides information about a different effect size.6
RESULTS
The literature search yielded 1,466 case reports. After careful screening against the eligibility criteria, a total of 751 case reports were included in the meta-analysis (Figure 1). There were 380 (45.67%) males, 334 (50.6%) females, and in 28 cases (3.73%) no gender identity was revealed. There was no history of drug allergy in any case reports. The mean age of males was 55.1 years (interquartile range: 41–68; range: 2–98), and of females was 53.1 years (interquartile range: 41–68; range: 2–98).
In 361 cases, the mechanism of DITP was drug-dependent antibodies, followed by autoantibody induction in 124 cases, drug-specific antibody in 106 cases, bone marrow suppression or toxicity in 72 cases, fibrinogen receptor antagonist-dependent antibody in 68 cases, and hapten-dependent antibody in 20 cases (Table 1).
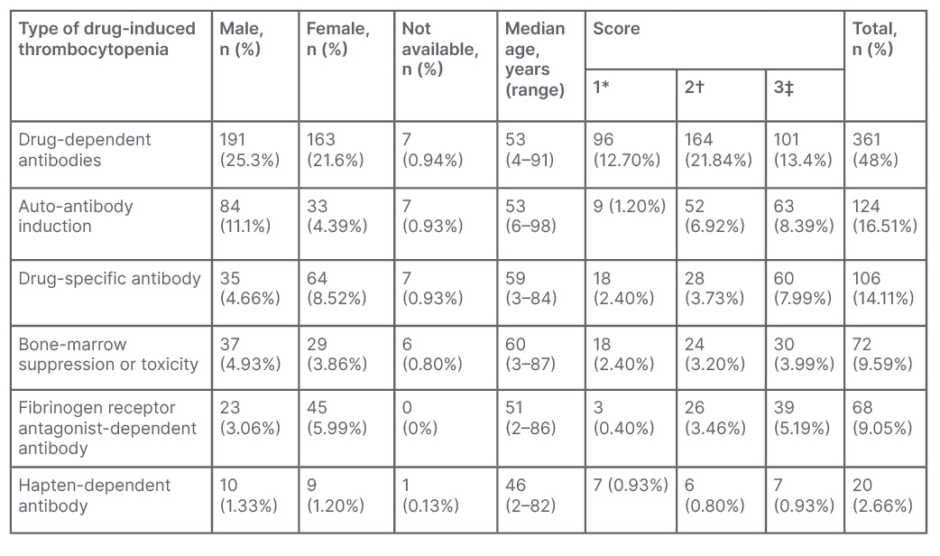
Table 1: Percentage and number of case reports on the basis of mechanism of drug-induced thrombocytopenic purpura.
*Thrombocytopenia definitely caused by drug .
†Thrombocytopenia probably caused by drug.
‡Thrombocytopenia possibly caused by drug.
Out of 751 articles, the score of 151 articles was 1, followed by 300 articles with a score of 2, and 300 articles with a score of 3. The incidences for all case reports score DITP by random and common effect model were 0.65% (95% CI: 0.61–0.69) and 0.65% (95% CI: 0.62–0.68) respectively.
In 151 patients with score 1, synthetic results through meta-analysis using the random and common effect models revealed an incidence of 0.16% (95% CI: 0.9–0.27) and 0.20% (95% CI: 0.17–0.23), respectively (Figure 2A). The total number of DITPs with Score 2 was reported in 300 patients, and synthetic results through meta-analysis using the random and common effect model revealed an incidence of 0.37% (95% CI: 0.31–0.44) and 0.40% (95% CI: 0.37–0.43), respectively (Figure 2B). The total number of DITPs with a score of 3 was reported in 300 patients, and synthetic results through meta-analysis using the random and common effect model revealed an incidence of 0.45% (95% CI: 0.35–0.55) and 0.40% (95% CI: 0.37–0.43), respectively (Figure 2C). Case reports of DITP scoring 4 and 5 were not included in this systematic review and meta-analysis.
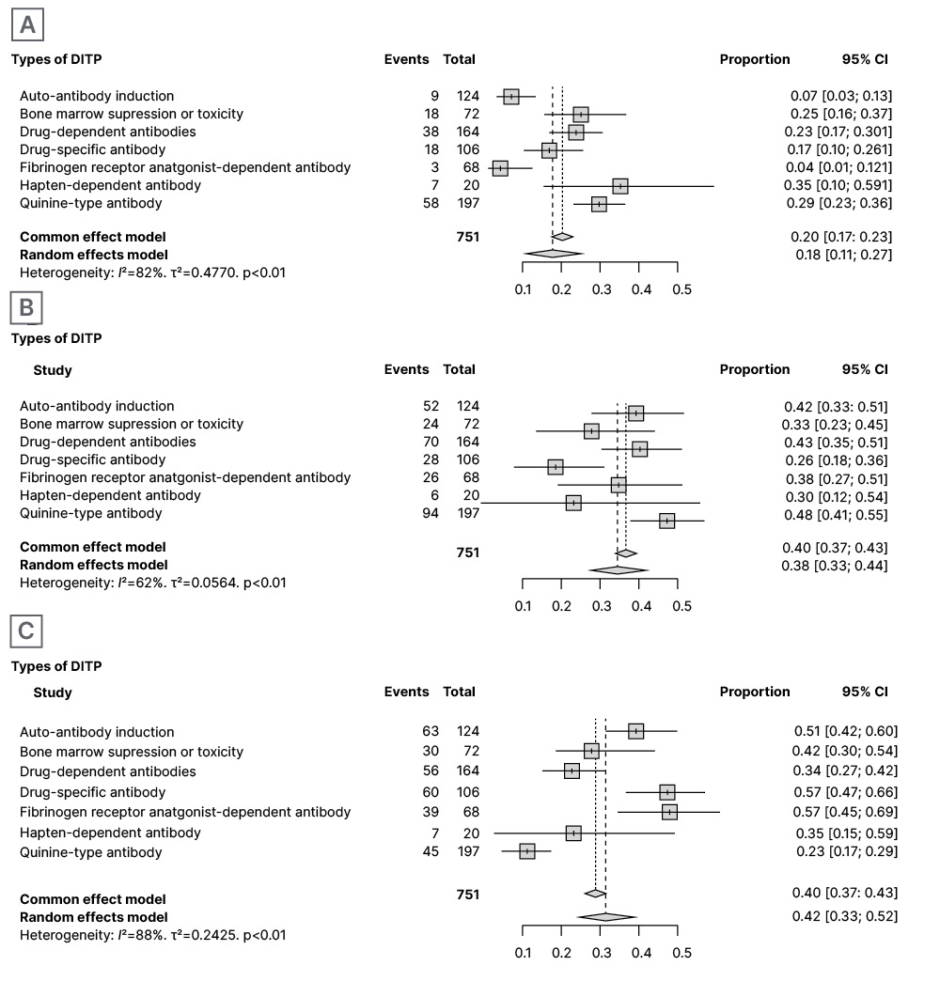
Figure 2: Proportion of all-article score of 1 out of total definitive incidences (A); proportion of all-article score of 2 out of total definitive incidences (B); and proportion of all articles score of 3 out of total definitive incidences (C).
CI: confidence interval; DITP: drug-induced thrombocytopenic purpura.
In 20.8% of the case reports, antibiotics caused DITP, followed by antimalarials in 13.7%, monoclonal antibodies in 10.1%, antiplatelets in 9.0%, DMARDs in 8.16%, anti-epileptics in 6.98%, anti-cancer chemotherapeutics in 6.45%, NSAIDs in 4.61%, H2-blockers in 3.95%, antiarrhythmics in 3.82%, antipsychotics in 2.23%, diuretics in 2.10%, anti-hypertensives in 1.97%, and vaccines in 1.18%. Other miscellaneous drugs were responsible for 4.74% of DITP.
On further analysis of the incidence of DITP, the authors found that out of these 751 patients, 478 (63.6%) patients required hospitalisation.The incidence through meta-analysis was 0.64% (95% CI: 0.52–0.75) and 0.64% (95% CI: 0.60–0.67), respectively. Out of these 478 hospitalised patients, 323 (67.5%) patients had external or internal bleeding, including 120 (37.1%) with trivial bleeding, i.e., petechiae, purpura, brief epistaxis or gingival bleeding, guaiac-positive stool, or microscopic haematuria; and 141 (43.6%) patients showing minor bleeding or overt bleeding that did not meet the criteria for major bleeding (melaena, gross haematuria, epistaxis, or gingival bleeding that is prolonged for more than 30 minutes and required medical intervention; or excessive menstrual bleeding or vaginal bleeding other than menses). Further, 62 (19.1%) patients showed major bleeding intracranially or retroperitoneally, or overt bleeding, which was visible or symptomatic, with a lab-confirmed decrease in haemoglobin concentration by more than 2 g/dL, or requiring transfusion of two or more units of red blood cells. The incidence through meta-analysis was 0.62% (95% CI: 0.48–0.75) and 0.68% (95% CI: 0.63–0.72), respectively.
Additionally, a total of 12 deaths were reported, with the following medicines being identified as the cause: quinine resulted in the death of five patients; antibiotics rifampin, trimethoprim, sulfamethoxazole, and vancomycin resulted in the death of three patients; followed by oxaliplatin in two patients; eptifibatide in one patient; and phenytoin in one patient.
Through meta-analysis, the incidence was 0.03% (95% CI: 0.01–0.05) and 0.03% (95% CI: 0.01–0.04), respectively.
PUBLICATION BIAS
In this systematic review and meta-analysis, publication bias was not assessed as the case reports included were not comparable for age, sex, causative drugs, drugs classification, or relation between causative drugs and clinical subtypes.
DISCUSSION
In literature, there have been case reports or case series of DITP. To the best of the authors’ knowledge, this is the first systematic review and meta-analysis to analyse DITP case reports and its mechanism.
In the present systematic review and meta-analysis, drug-dependent antibodies-induced DITP was the most frequent mechanism, which accounted for 48% of the case reports. This mechanism has been explained as an immune-mediated reaction where drug-specific antibodies are derived from pre-existing, naturally occurring antibodies in the body that have a weak affinity for platelets in the absence of causing drugs.7 Once the sensitising drug is introduced into the body, the drug binds to platelet-specific GP IIb/IIIa or Ib/V/IX complexes. It may also interact with an intact integrin and result in conformational changes that further increase antibodies’ affinity for the platelet membrane GP. The arginase 1-10 residue in the GP IX sub-unit also plays a critical role at the antigenic site.8,9
In this meta-analysis, quinine, quinidine, and antibiotics such as sulfamethoxazole, vancomycin, and β-lactams, anti-epileptics drugs; NSAIDs, H2-blockers, and proton-pump inhibitors were reported to cause DITP by this mechanism. The onset of drug-dependent antibodies-related manifestations approximately takes 1–2 weeks after initiation of drug therapy. However, it has been reported that antibodies often emerge over a protracted period of intermittent drug usage.2 One retrospective analysis reported vancomycin-specific platelet-reactive antibodies in 20% (n=34) samples.10
Additionally, they also reported the median time to platelet nadir (mean: 13,600 per mm3) 7 days and 93% average fall in platelet counts. Ten patients presented with severe bleeding in the form of haematuria, intrapulmonary and gastrointestinal haemorrhages, and excessive bleeding from the site of the vein punctured. During acute thrombocytopenia, 14 patients who received platelet transfusions did not show a rise in platelet counts. The sole beneficial strategy for these patients was the discontinuation of vancomycin.
In this meta-analysis, autoantibody-dependent was the second most commonly reported mechanism for DITP, which was identified in 16.5% of case reports and demonstrated by DMARDs, interferon-α procainamide, and levodopa.11,12 The exact mechanism behind this autoantibody-mediated reaction is not understood clearly. However, laboratory and clinical data suggested that auto-antibodies are produced only in the presence of these drugs. Another proposed mechanism is an interference of the drug with platelet surface GP and the formation of unknown peptides, which further stimulate the removal of platelets by the immune system.8
The third reported mechanism for DITP was drug-specific antibodies, described in 14.1% of case reports. Abciximab has been reported to induce DITP in this manner. Abciximab, a chimeric (human/mouse) monoclonal antibody is used as an antiplatelet agent, and acts by binding to the anti-GP IIb/IIIa Fab fragment that blocks the binding of fibrinogen to platelet and inhibits thrombus formation.13 After receiving abciximab, approximately 10–15% of patients may develop thrombocytopenia within 30 days due to antibody formation.14 However, antibodies already present in blood circulation cause acute thrombocytopenia in 1–2% of patients exposed to abciximab for the first time, and 10–12% of patients treated for the second time with this drug.9 Usually, bleeding manifestations are transient, but life-threatening intracranial haemorrhages have also been reported in many patients. IgG/IgM antibodies recognise murine sequences present in the abciximab molecule for its binding to GP IIb/IIIa, whereas non-pathogenic antibodies are specific for the papain-cleaving site of abciximab Fab fragment (not associated with platelet depletion).12 In the authors’ included case reports, most of the patients recovered within a few days, but in some patients, low platelet counts persisted for several weeks. On the other hand, delayed thrombocytopenia was also observed in a few patients after 5–10 days of treatment with abciximab, with a decrease in platelet count. This can be explained by the possibility that abciximab may be present for up to 2 weeks in circulating platelets because of the movement of the drug from one cell to another.13 Therefore, delayed abciximab-induced thrombocytopenia could be caused by newly synthesised antibodies against the drug remaining on the surface of the platelets for an extended period. This DITP usually occurs after hospital discharge and may be severe, with a delayed diagnosis. The condition becomes more severe in patients who are on treatment with other antiplatelet drugs, such as aspirin or P2Y12 inhibitors, favouring the occurrence of severe bleeding.14
The TARGET study randomised 4,809 patients and reported abciximab or tirofiban induced thrombocytopenia in 2.9% of patient undergoing percutaneous coronary intervention.15,16 The percentage of DITP was significantly higher in abciximab in comparison to patients who received tirofiban (2.5% versus 0.5%). The study also reported lower mean nadir platelet count, higher incidence rate of profound thrombocytopenia, and longer mean days to platelet recovery in patients receiving abciximab than tirofiban. Several humanised monoclonal antibodies have also been identified as potential triggers for the formation of autoantibodies targeting platelets. Monoclonal antibodies causing DITP include efalizumab (anti-CD11a),17,18 adalimumab,19 infliximab (anti-TNF), bevacizumab (anti-vascular endothelial growth factor therapy),20,21 rituximab (anti-CD20),22,23 natalizumab (anti-4 1-integrin),24 and programmed death-1 or cytotoxic T-lymphocyte antigen 4.25,26
In the present meta-analysis, drugs causing DITP by bone marrow suppression or toxicity accounted for 9.5% of DITP cases. It is a drug-induced, non-immune mediated manner of thrombocytopenia; drugs induce myelosuppression, followed by decreased production of platelets. Oxaliplatin was the most common drug reported to cause thrombocytopenia in this manner. Based on several reports, 45–77% of patients with colorectal cancer who received oxaliplatin developed thrombocytopenia.1,16,27 Bleeding was not the presenting symptom in these cases, but concomitant anaemia and neutropenia were frequent. Lower mean nadir platelet is reached within 10–14 days after drug administration.7 The other drugs reported to cause DITP by this mechanism include β-lactams, linezolid, sulphonamide, flucytosine, ganciclovir, valganciclovir, foscarnet, and albendazole.28,29
Fiban-type drug reaction is another mechanism of DITP (9.05%). Antiplatelet drugs eptifibatide and tirofiban, followed by vaccines, captopril, and amphotericin B were the most commonly reported drugs found to be associated with this mechanism of DITP. Ligand-mimetic fibrinogen-receptor antagonists mimic the arginine-glycine-aspartic acid sequence recognised by specific sites of the platelet GP IIb/Illa complex. They inhibit fibrinogen-GP lIb/llla interactions competitively, and prevent the formation of platelet aggregates. Various studies reported tirofiban and eptifibatide as the most commonly reported compounds used to prevent thrombotic complications associated with percutaneous transluminal coronary angioplasty, and approximately 0.1–0.5% of patients may develop thrombocytopenia when treated first with these drugs. This adverse event was reported to develop in some patients within the first 24 hours of treatment, with clinical complaints of fever, chills, and hypotension.30 However, most of the patients recovered within a few days without severe bleeding. Platelet-sensitive antibodies recognise a neoepitope or a ligand-induced binding site expressed by GP IIb/Illa to cause platelet destruction.29 Eptifibatide-dependent antibodies do not bind to GP Ilb/Illa in the presence of tirofiban, and vice versa.
These antibodies may be present naturally without any previous history of drug exposure.31,32
Hapten-dependent antibodies mechanism of DITP is 2.66%. Haptens are small molecules and are themselves not immunogenic, but once they bind covalently to macromolecules like proteins, they synthesise drug-specific antibodies against platelets. This mechanism was reported with penicillin and cephalosporin drugs, which may bind to red blood cells via their β-lactam ring, and induce immune haemolytic anaemia. Another mechanism for immune-mediated thrombocytopenia may be the covalent binding of penicillin or their derivatives with platelet GPs and induction of antibody response.33-35 Cephalosporins (mainly ceftriaxone) have been reported to cause DITP, with antibodies recognising GP llb/llla or GP Ib/IX, especially the GP IX sub-unit.36
The overall mortality of patients with DITP was 1.5% in the meta-analysis. Death rate with drug-dependent antibodies type appeared to be higher in quinine recipients. Allergic reactions to quinine can be severe, and can cause severe multi-organ failure. Early recognition is critical for the prevention of recurrent episodes.
CONCLUSION
In this systematic review and meta-analysis, the authors found that antibiotics, antimalarials, monoclonal antibodies, antiplatelets, DMARDs, anti-epileptics, and anti-cancer chemotherapeutic drugs were commonly associated with DITP. Clinicians should be aware of the potential of drugs causing DITP as an adverse event, which may affect patient compliance and adherence to drugs. Unrecognised DITP may also lead to severe thrombocytopenia and inappropriate patient management.





