Abstract
Stinging insects that cause allergic reactions belong to the order Hymenoptera, which includes wasps, hornets, bees, yellow jackets, true hornets, and stinging ants. Individuals stung by these insects can have different clinical outcomes, from common local reactions to severe systemic reactions. Anaphylaxis as a result of insect stings can result in death; therefore, individuals with a history of systemic reaction to stings should be further evaluated and treated. A history of systemic reaction to insect stings and immunoglobulin E sensitivity to specific insect venoms, determined by blood or skin testing, are criteria for venom immunotherapy administration. Venom immunotherapy modulates the immune system to make the recipient less sensitive to venom and can be curative. All individuals with a history of systemic reaction to insect stings should be provided with an adrenaline auto-injector and educated in avoidance measures to prevent future stings. This review will discuss the diagnosis of venom allergy, the management of venom allergic individuals with venom immunotherapy, and identification of risk factors for severe anaphylaxis to insect stings. This review will also aid clinicians in discussing avoidance measures with patients.
STINGING INSECT CHARACTERISTICS
Stinging insects that cause allergic reactions belong to the order Hymenoptera, which contains three medically important families: Apidae, Vespidae, and Formicidae. The family Apidae includes honey bees, bumblebees, and sweat bees; the family Vespidae includes yellow jackets, yellow hornets, bald-faced hornets, true hornets, and paper wasps; and the family Formicidae includes the fire ant, jack jumper ant, and harvester ant. Most individuals are not able to properly identify the culprit insect after a sting; therefore, knowing certain characteristics of these stinging insects can aid in the identification. For instance, the worker honey bee has a barbed stinger that usually remains in the skin after a sting, although it should be noted that sometimes the stinger from other insects remains in the skin, especially if swatted. Yellow jackets usually nest underground, while hornet nests are found in bushes, trees, and overhangs. Yellow jackets are scavengers and tend to fly around rubbish bins and food. Paper wasps build small, open celled nests that hang from a pedicle on eaves or in shrubs. Wasps are attracted to open bodies of water, e.g., swimming pools and streams. Fire ants are prevalent in the south eastern USA, Central and South America,1,2 and have spread to Hong Kong and Japan.3 Fire ants build their nests as mounds in the ground and use their mandibles to hold onto their prey as they administer multiple stings. The jack jumper ant, Myrmecia pilosula, is prevalent in Tasmania and mainland Australia.4 Features such as these can aid the clinician in history taking and insect identification, as well as educating patients on avoidance measures, which will be discussed later in the review (Table 1).
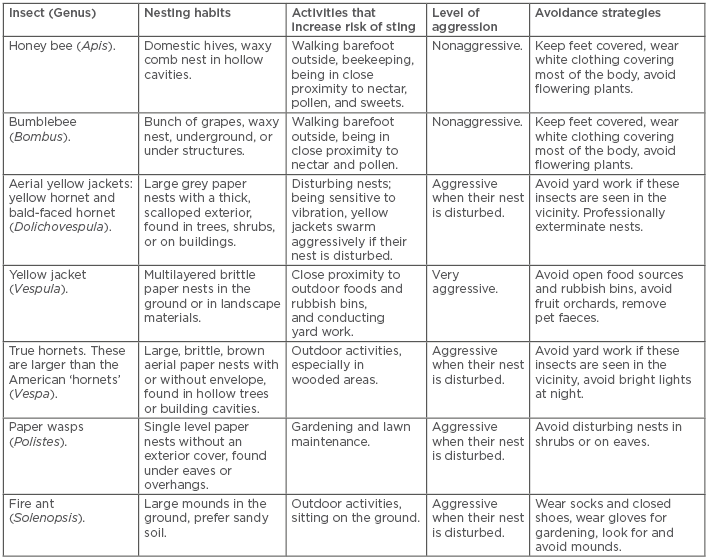
Table 1: Hymenoptera characteristics and avoidance measures.
CLINICAL SYMPTOMS
In general, reactions to insect stings fall into two categories: local and systemic.
Local Reactions
Most stings will result in an immediate-type local reaction that lasts for a few hours. Local reactions are raised, erythematous, and pruritic. About 5–25% of individuals will experience large local reactions (LLR).5 LLR usually occur hours after the sting and manifest clinically as an area of erythematous induration surrounding the sting site.6 LLR can measure >10 cm in diameter and can cross joint lines.6,7 This area is usually painful and pruritic, which can last for several days after the sting. LLR are usually an immunoglobulin (Ig)E dependent late-phase reaction, and patients who experience a LLR have about a 4–10% risk of a systemic reaction to a future sting.8 The European Academy of Allergy and Clinical Immunology (EAACI) Task Force on Venom Immunotherapy reports a 0.8–7.0% risk of systemic reaction to future stings in those with a previous LRR.7 Fire ant stings cause a sterile pruritic pustule at the sting site(s), which typically develop about 24 hours after the sting.
Systemic Reactions
Stinging insects can cause IgE-mediated systemic reactions that are cutaneous-only or life threatening.9 Life threatening systemic reactions that involve one or more organ systems are referred to as anaphylaxis. Anaphylaxis can have several different manifestations: respiratory compromise resulting in stridor, wheezing, coughing, or shortness of breath; cardiovascular involvement resulting in hypotension, lightheadedness, syncope, or arrhythmia; gastrointestinal upset, including nausea, vomiting, abdominal cramp and pain, or diarrhoea; skin findings, such as flushing, pruritus, urticaria, and angioedema; and neurological symptoms, including seizures, or a sense of impending doom. All of the aforementioned are signs and symptoms of this condition. In about 60% of children with allergic reactions to Hymenoptera stings, skin symptoms are the sole manifestation, but this is found in only 15% of adults.10 In the USA, 3% of adults and 1% of children are reported to have had a systemic reaction to an insect sting.5 In Europe, the prevalence of systemic reactions due to stinging insects is 0.3–7.5%.6 At least 40 fatal sting reactions occur in the USA10,11 and about 20 fatal reactions from stings occur in the UK annually.12 Patients with a prior systemic reaction to a sting have about a 30–65% risk for a subsequent systemic reaction to future stings, even to the same species.10 The majority of deaths occur in adults as a result of circulatory collapse. Turner et al.13 report a summary of 535 stinging insect fatalities that showed 80–90% of fatal cases occurred in men with an average age of 50–60 years. Half of the fatal reactions that occur with stinging insect allergy have no history of sting reaction.10 There appears to be a higher risk of having a systemic reaction if there are multiple stings at the same time, or if repeated stings in a single season occur.14
In addition to IgE-mediated reactions, stinging insects can cause adverse clinical outcomes due to envenomation from a large number of stings. Envenomation causes systemic toxicity due to the increased load of venom in the body. Toxic compounds found in venom such as phospholipase A1, hyaluronidase, and antigen 5 can be toxic to tissues in the body.15 Venom can also trigger proinflammatory cytokines such as IL-1β, TNF-α, and IL-6, which has been demonstrated in mouse models.16 Acute renal failure, hypotension, haemolysis, and rhabdomyolysis have all been described in the literature as causing adverse reactions due to envenomation.17 Fire ant venom contains alkaloids that are capable of causing cardiorespiratory depression and seizures.18
Other Findings
Mast cell disease is reported in 1–8% of Hymenoptera venom allergic patients in Europe,19 and is reported in 2% of Hymenoptera venom allergic patients in the USA.8,19 These patients usually have an elevated basal serum tryptase (BST) level (>11.4 ng/mL) and may develop more severe anaphylaxis to Hymenoptera stings, even with the first sting. They may have severe hypotension and loss of consciousness without cutaneous findings and have a higher risk for mortality from Hymenoptera stings.10 Other uncommon reactions reported after Hymenoptera stings include serum sickness-like reactions and chronic urticaria.5,10
DIAGNOSIS
Diagnosis of venom allergy is based on the clinical history and identification of venom specific IgE to the culprit insect by skin testing or in vitro tests. Only patients with a history of anaphylaxis to stings or individuals >16 years of age with diffuse cutaneous reactions need to undergo skin testing or in vitro tests.8 In the USA, five Hymenoptera venoms are available for testing: honey bee, yellow jacket, yellow hornet, bald-faced hornet, and paper wasp. Skin testing is usually done with skin prick tests at 1-100 µg/mL concentration. A German article advocated use of up to 300 µcg/mL venom for skin prick testing.20 If prick tests are negative, then intradermal skin tests are done at progressively increasing concentrations from 0.001–0.010 µg/mL (increase by 10-fold increments), until either a positive skin test occurs or the 1 µg/mL concentration is reached.8 Quirt et al.21 advocate using a single step protocol with intradermal skin testing with 1 µg/mL of the five commercially available venoms without preceding skin prick testing or having a stepwise approach, but this has not been adopted by North American or British guidelines.8,22 For imported fire ants, a whole body extract is used for testing. Skin prick testing with 1:1000 weight per volume (w/v) with an imported fire ant body extract is carried out, then, if negative, intradermal skin tests starting with 1:1 million w/v should proceed and increase at 10-fold increments until there is a positive test, or the 1:1000 w/v concentration is reached. A positive skin prick test response is usually defined as a wheal of 3 mm or greater than the negative control, whereas a positive intradermal skin test is a wheal 5 mm or greater than the negative control. Venom skin testing is positive in 70–90% of patients with a clinical history of Hymenoptera sting allergy, but about 25% of patients react only to the 1 µg/mL intradermal skin test.10 If skin testing is negative, then in vitro testing should be carried out that, if positive, confirms the diagnosis. The level of specific IgE antibody to venom does not predict the severity of the allergic reaction to a sting.23 If both skin testing and in vitro testing are negative in a patient with a history of sting anaphylaxis, the skin testing should be repeated after 6–12 weeks.
There are some patients for whom testing to whole venom extracts will not identify the cause of the allergy. About 4–6% of patients with a history of systemic reaction to Hymenoptera stings have negative skin tests and in vitro tests to whole Hymenoptera venom.24 Another problem is that some patients are sensitive to multiple Hymenoptera venoms.7 In vitro testing with recombinant venom proteins may provide information as to whether allergy to multiple different venoms is due to cross-reactivity of IgE to similar venom proteins, or if there is true double sensitisation to unique proteins in different types of venom. Molecular component tests are available in Europe to recombinant proteins for honey bee (Apis), paper wasp (Polistes), and vespid (Vespula) venoms. The most commonly used are the antigen 5 proteins (Pol d 5 and Ves v 5), the phospholipases (Api m 1, Pol d 1, and Ves v 1) and bee acid phosphatase (Api m 3), melittin (Api m 4), and icarapin (Api m 10).25 Use of these recombinant venom proteins allows the determination of which patients are sensitised to multiple venoms versus those who demonstrate cross-reactivity.25 For instance, component testing may be helpful in evaluating those individuals who have positive specific IgE against both honey bee and yellow jacket venom. Those patients who are positive to Api m1, Api m3, and/or Api m10 should receive honey bee venom immunotherapy (VIT); patients who are positive to Ves v1 and/or Ves v5 should receive yellow jacket VIT; and individuals positive for both Api and Ves components should receive honey bee and yellow jacket VIT.26 Studies have shown that double positive IgE to component proteins occurs in about 50% of vespid allergic cases.24 Bumblebee venom is available for in vitro testing but is not available for skin testing or treatment. Bumblebee venom has limited cross-reactivity to honey bee venom.8
MANAGEMENT AND THERAPEUTICS
Supportive Care Techniques
Uncomplicated local reactions to stings are treated with cold compresses and oral antihistamines. Urticaria is treated with oral antihistamines. Cold compresses, oral antihistamines, high potency topical steroids, nonsteroidal anti-inflammatory drugs for pain, and oral steroids for more extensive and persistent swelling are all suitable treatments for LLR.
Treatment of Anaphylaxis
Anaphylaxis is initially managed with intramuscular adrenaline, which is usually injected into the anterolateral thigh and additional doses may be required. Full emergency medical care should be given. The patient should be placed in a supine position with the legs elevated to promote blood return to the heart.10 Hypotensive patients should be given isotonic intravenous fluid. Oxygen is given if oxygen saturation is low, and a bronchodilator is administered for persistent bronchospasm; intubation and mechanical ventilation may be required. Patients should be monitored for prolonged or recurrent anaphylaxis. Glucagon should be prescribed to patients taking a beta-adrenergic blocker who have hypotension.10
Venom Immunotherapy
It is important to know that only those that have experienced a systemic allergic reaction to an insect sting should receive further testing to see if they are a candidate for VIT. Patients with positive results should be counselled about VIT, and it should be initiated if possible. VIT is highly effective and may be curative therapy for many with IgE mediated venom allergy.5,7 The principle of VIT is to introduce small amounts of venom to the patient, and, by gradually increasing increments of venom administration, modulate the immune system, which makes the individual less sensitive. For flying Hymenoptera insects, VIT is made from pure insect venom. It is difficult to obtain enough pure venom from fire ants to offer it commercially; as a result, the whole body of the fire ant is crushed to create extracts for diagnosis and treatment.27 Fire ant whole-body extracts contain sufficient venom proteins for therapeutic use but are inferior to purified ant venom for testing and treatment.28 Mixed vespid VIT is 95–100% effective at preventing systemic reactions to future stings, but bee venom is less effective, about 77%.5,7 VIT has a similar safety profile to aeroallergen immunotherapy. Systemic symptoms to VIT occur in 5–15% of the population during their first weeks of treatment and are usually mild.5
Vespa crabro is a prevalent stinging insect capable of causing anaphylaxis and is prevalent in the Mediterreanean area of southern Europe. Most proteins in Vespa and Vespula families are highly cross-reactive; however, the antigen 5 from Vespa crabro and Vespula are unique and not cross-reactive, so treatment with Vespula VIT will not confer complete protection.29 Vespa crabro venom is available for testing and treatment in some European countries.
VIT schedules can vary but typically an individual will receive subcutaneous injections weekly until a maintenance dose is achieved on a standard schedule. Once a maintenance dose is achieved, patients receive monthly therapy for at least 1 year. Following this, the maintenance interval is extended to every 6–8 weeks.5 VIT is usually administered for 3–5 years.7 Patients needing maintenance therapy for >5 years may be given VIT at 12 week intervals.8 Certain scenarios would cause an individual to be on injections indefinitely, such as those with a honey bee allergy. Patients with a mast cell disorder or those who had a systemic reaction to a sting while actually on VIT may also need treatment >3–5 years.5 This is best addressed on a case by case basis. There are schedules available to help reach maintenance dosing faster such as rush and ultra-rush, in which maintenance dosing can be achieved in days. It is reported that ultra-rush scheduling is associated with a higher risk of systemic reaction to VIT.8,7
The specific venom used for treatment depends on the history of the culprit insect. However, as many patients are unable to identify the specific type of insect causing the sting, there is debate as to whether treatment with VIT should include all venoms with positive tests or only the most likely culprit insect venom. Use of in vitro recombinant venom component testing may help to resolve this issue in the future.
Although this assay is not yet standardised, basophil activation tests (BAT) may be useful in determining if VIT has been successful at achieving a protective immune response.7 BAT use basophils from a patient with suspected Hymenoptera allergy, and these basophils are exposed to defined concentrations of Hymenoptera venom. Activation is measured based on the percentage of basophils that express the activation marker CD63 on basophils.8 EAACI states that performing sting challenges is the most reliable and gold standard for monitoring the effectiveness of VIT. Sting challenges are used to identify those individuals who are not protected at the maintenance dose of 100 μg.7 Sting challenges are not routinely performed in the USA.
Contraindications to Venom Immunotherapy
The EAACI Task Force on Contraindications to Allergy Immunotherapy recommends several absolute contraindications to VIT: poorly controlled asthma, active autoimmune disorders, active malignant neoplasias, children <2 years of age, and acquired immune deficiency syndrome (AIDS).30 Use of angiotensin-converting-enzyme (ACE) inhibitors and beta-blockers are not a contraindication for VIT.7,30 Pre-existing cardiovascular disease, even in elderly patients, is not a contraindication to VIT.7
Identifying Risk Factors for Anaphylaxis
Certain risk factors can put an individual at increased risk of severe anaphylaxis or death as a result of an insect sting. Experiencing anaphylaxis without cutaneous symptoms (such as urticaria or angioedema) is a risk factor for severe reaction with future sting.31 Older age (>45 years), chronic cardiovascular disease, and having a history of a previous severe anaphylactic reaction are also risk factors for further anaphylaxis.5 There is controversy as to whether patients taking beta-blockers or ACE inhibitors may have some increased risk of anaphylaxis, because they may be less responsive to adrenaline treatment and require repeat dosing.8 However, currently the United States Joint Task Force practice parameters advise that patients requiring beta blockers or ACE inhibitors should continue VIT, as the benefits of VIT outweigh the risk of severe reactions to Hymenoptera stings.8 Honey bee venom is associated with a higher risk for reaction during the build-up phase of VIT and has a lower rate of protection from future sting reactions (85% for honey bee versus 96% for mixed vespid venom).32 Patients who were not protected at the usual maintenance dose of 100 µg of honey bee or vespid venom were protected when the maintenance dose was increased to 200 µg.32
Individuals with a clonal mast cell disorder or elevated baseline tryptase may also be at an increased risk for severe reactions to VIT and Hymenoptera stings.7,8 BST level should be measured after the reaction has fully resolved in all patients with anaphylaxis to Hymenoptera stings, typically about 4–6 weeks after the reaction. Patients with a BST level >11.4 ng/mL should undergo an evaluation for an underlying mast cell disease; BST levels of 20 ng/mL or higher are a minor criterion for diagnosis of clonal mast cell disease. Patients with elevated BST levels also have a higher risk of systemic reactions to VIT injections, and increased risk of treatment failure with VIT.10 About 15% of patients with mast cell disease will have systemic reactions to Hymenoptera stings but negative tests for IgE to venom.24 However, patients with mastocytosis and evidence of allergy to Hymenoptera stings should receive VIT.7
Avoidance Measures
Avoiding cooking and eating outdoors, walking outside barefoot, and exposure to flowering plants can help decrease the risk of encountering stinging insects.8 If an individual encounters a flying Hymenoptera insect, they should slowly walk away from the area and avoid swatting at the insect or making sudden movements; furthermore, stinging insects are attracted to an individual’s breath, so covering the mouth and nose is recommended. Seeking shelter indoors or in an enclosed vehicle should be pursued if possible. Fire ants may sting multiple times and should be quickly removed if found on the skin. Fire ant mounds should be treated with pesticides when seen. Hymenoptera are most defensive near their nests, so observing the area for flying insects or visible nests prior to starting outdoor activities can also aid in avoidance. (Table 1)
SUMMARY
Stinging insect allergy can result in different clinical manifestations. Those with systemic reactions require prompt treatment with adrenaline and the clinician should educate these patients on avoidance measures to prevent future stings. Individuals with systemic reactions to insect stings should undergo further testing so the diagnosis of stinging insect allergy can be made. If so, these patients could be candidates for VIT, which can be curative.






