Omalizumab is a recombinant human monoclonal antibody that blocks the immunoglobulin (Ig)E receptor and is indicated in cases of severe asthma (SA) and chronic idiopathic urticaria (CIU).1,2 Clinical responses to omalizumab seem to be different in SA and CIU;3,4 therefore, the aim of this study was to demonstrate the different effect omalizumab has on SA and CIU.
A pulmonary function test, skin prick test with aeroallergens (ALK, Hørsholm, Denmark), serum specific-IgE (ImmunoCAPTM, Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA), and asthma control test were performed. Follow-up data of 49 patients, who were treated with omalizumab between March 2014 and June 2017, were evaluated 1 year after treatment. Out of the 49 patients, 23 were re-evaluated during this year (11 females). Most of the treated patients had CIU (n=38), while 23.1% (n=11) had SA; these figures almost doubled compared to 1 year ago (20 and 6 patients with CIU and SA, respectively). In patients with SA, omalizumab was given according to the patient’s total IgE level and body weight, whereas in CIU a dose of 300 mg/month was used.
The responses of CIU and SA to omalizumab are shown in Table 1. Although the duration of CIU in patients was similar after 1 year, the duration of SA was significantly less 1 year after treatment (25.33±10.38 years and 16.90±3.81 years, respectively). Treatment compliance was significantly better in both groups after 1 year, despite the duration of omalizumab treatment remaining unchanged. Almost half of the CIU patients showed complete remission in both evaluations, while the number of cases of complete remission observed in the SA patients after 1 year (18.2%) was significantly increased compared to the previous year (0.0%; p<0.05). The majority of patients with CIU (>80%) had used antihistamine or leukotriene receptor antagonist therapeutics, whereas all patients with SA had used either inhaled corticosteroids or long-acting beta-agonists, six individuals had received additional oral steroids (compared to only one patient 1 year ago). However, 1 year ago, out of a total of 26 patients enrolled, 20 (76.9%) had CIU, with a female predominance of 53.8%.
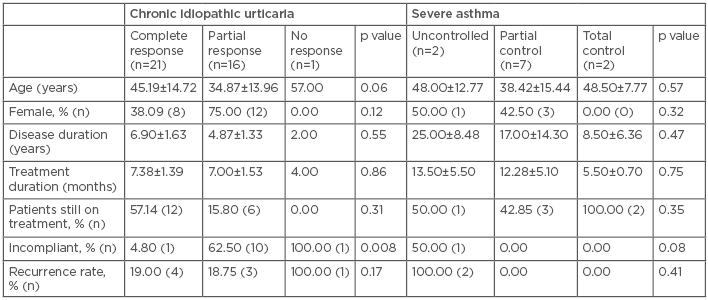
Table 1: Comparison of response to omalizumab in chronic idiopathic urticaria and severe asthma.
Treatment failure dropped to almost half of the SA patients, but no change was observed in the CIU cohort. In addition, the recurrence rate following discontinuation of omalizumab was almost half in CIU patients (from 43.8% to 23.7% after 1 year) and improved even more dramatically in SA patients (from 83.3% to 18.2% after 1 year; p<0.01), which shows the power of compliance. No variables were related with remission, treatment failure, or recurrence.
Therefore, omalizumab appears to be more effective in CIU patients during the first year of treatment, whereas treatment of SA seems to improve equally in the first and second year. However, as omalizumab treatment continues, not only the treatment failure rate and the remission rate of the disease improve, as shown by an asthma control test, but, maybe more importantly, recurrence of SA is not seen. In conclusion, although quick relief and response to omalizumab in CIU seems to be an advantage for patients, this leads to less compliance and more disease recurrence.






