BACKGROUND
Over the past two decades, new opportunities have emerged in the treatment of severe bronchial asthma due to the development of immunobiological therapy. The effectiveness of biologics depends on correct phenotyping of asthma in patients.1 The Phadiatop test has been known since the 1980s and has established itself as a screening test for the detection of atopy, allergic rhinitis, and allergic asthma.2-9 When selecting patients with severe bronchial asthma for targeted therapy in the Sverdlovsk region, Russia, the Phadiatop™ (Phadia AB, Uppsala, Sweden) screening test was used for phenotyping asthma in this group of patients.
AIM
To assess the informative value of the Phadiatop test when selecting patients with severe bronchial asthma for target therapy.
MATERIALS AND METHODS
In the course of work on a regional register of patients with severe asthma (SA), patients were selected and asthma was phenotyped. Allergic anamnesis, skin tests with allergens, specific IgE to allergens, and blood eosinophil level were used as standard methods for asthma phenotyping. The Phadiatop test has been used since 2016 to screen for allergic phenotypes of SA when selecting patients for target therapy. The registry included 77 patients at the time of the cross-sectional study (January 2021). The study analysed data from all patients in the registry. The diagnosis of allergic SA was established with a positive allergic anamnesis (there is a connection between the clinical symptoms and exposure to allergens) and at least one positive test (skin tests, specific IgE) confirming sensitisation.
RESULTS
According to phenotyping, the patients were divided into three groups: atopic SA J45.0 (n=43); non-allergic SA J45.1 (n=29); and mixed SA J45.8 (n=5). The result of Phadiatop testing in patients with atopic (3.86 PAU/L; Q1–Q3: 1.21–7.29) and mixed SA (2.36 PAU/L; Q1–Q3: 0.38–6.24) exceeded the results of this test in patients with non-allergic asthma (0.1 PAU/L; Q1–Q3: 0.04-0.24) 38.6 times (p=0.001) and 23.6 times (p=0.001), respectively (Table 1). Total IgE level analysis in phenotypic groups of asthma did not show significant differences (p=0.2).
The results of Phadiatop testing were compared with the results of standard diagnostic methods for atopy. In the group of patients with a positive allergic anamnesis, the result of the Phadiatop test was statistically higher than in the group of patients without an allergic anamnesis: 3.86 PAU/L (Q1–Q3: 0.89–7.00) and 0.12 PAU/L (Q1–Q3: 0.03–0.46), respectively (p<0.001). In the group of patients with positive skin tests, the result of the Phadiatop test was higher than the result in the group of patients with negative skin tests: 3.56 PAU/L (Q1–Q3: 1.27–6.79) and 0.07 PAU/L (Q1–Q3: 0.03–0.13), respectively (p=0.005). When comparing the results of Phadiatop testing in groups of patients with positive and negative specific IgE to allergens, statistically significant differences were also obtained: 2.64 PAU/L (Q1–Q3: 1.43–6.76) and 0.04 PAU/L (Q1–Q3: 0.02–0.07), respectively (p=0.022). The total IgE levels in the atopy and non-atopy groups did not differ significantly.
In the receiver operating characteristic analysis, threshold values were obtained at the cut-off point: with a Phadiatop test value >1.53 PAU/L, the patient is predicted to have a clinical reaction to the allergen (sensitivity: 72.4%; specificity: 94.4%) (Figure 1, Pictures 1 and 2) and positive skin tests (sensitivity: 75%; specificity: 100%); with a Phadiatop result >0.64 PAU/L, a positive result of specific IgE was predicted (sensitivity: 100%; specificity: 100%).
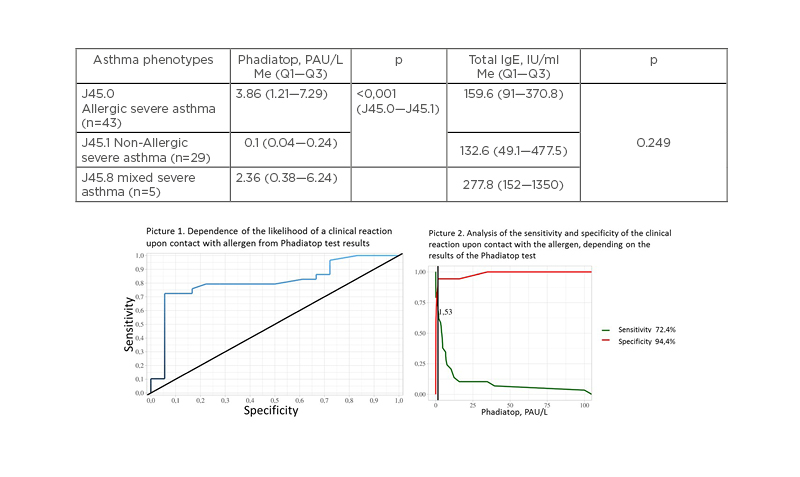
Figure 1: Phadiatop and total IgE results in different asthma phenotypes groups in register of severe asthma of Sverdlovsk region, 2016-2020.
CONCLUSION
It is advisable to use Phadiatop testing as a screening method for detecting the atopic phenotype of SA and selecting patients for immunobiological therapy.






