This European Academy of Allergy and Clinical Immunology (EAACI) presentation discussed the clinical development programme of a new B cell epitope-based allergy vaccine. The compound is based on fusion proteins, consisting of nonallergenic peptides derived from the immunoglobulin (Ig)E-binding sites of disease-causing allergens not binding IgE themselves, and PreS, a hepatitis B surface protein that serves as a nonallergenic carrier protein providing T cell help. The vaccine design and the three-dimensional structure of the Phl p 5 allergen was first described by Focke-Tejkl et al. in 2015.1 The clinical profile and efficacy of BM32 has been evaluated in four clinical double-blind, placebo-controlled trials.
The absence of IgE and T cell-mediated side effects, which are standard in extract-based immunotherapeutic preparations and an important cause of treatment discontinuation, has been demonstrated in a skin test study by Niederberger et al. in 2015.2 A total of 60 grass pollen-allergic volunteers exhibited an expected positive skin reaction in skin prick tests with grass pollen extract, but not with the individual BM32 components and the BM32 protein mix. T cell reactivity was investigated using two different concentrations of BM32. In contrast to grass pollen extract, no specific reaction was evident with BM32.
A dose-finding study conducted in an allergen challenge chamber system analysed clinical and immunological responses to three different BM32 doses in 69 grass pollen-allergic subjects.3 A dose of 20 µg or 40 µg of each BM32 component, compared to 10 µg of placebo, administered three times in monthly intervals, showed a significantly better efficacy compared to placebo in terms of reduction of total nasal symptom score, skin prick test reactivity, and induction of allergen-specific IgG4.
These results were confirmed by a consecutive multicentre, multinational study conducted in 181 allergic subjects over two consecutive grass pollen seasons.4 In this study, it was shown that the 20 µg dose was the most clinically effective in terms of combined symptom medication score as well as regarding quality of life during the pollen season. The induction of allergen-specific IgG was even more pronounced during the second year of treatment and no increases in IgE levels were recorded.
The dosing regimen was further evaluated in a single-centre study investigating the immunological and clinical response of 3, 4, or 5 monthly administrations of 20 µg of each BM32 component. A significant induction of allergen-specific IgG1 and IgG4 compared to placebo was achieved with all active dosing regimens in the full analysis set (N=120). The group receiving five preseasonal injections of BM32 developed the highest and most sustained allergen-specific IgG1 and IgG4 responses compared to the other groups (Figure 1). A dose-dependent reduction in total nasal symptom score for the 3, 4, and 5 monthly administrations of 42.9% (BM32 5x), 42.1% (BM32 4x), and 7.3% (BM32 3x), respectively, versus placebo were observed after the grass pollen season and upon exposure in a pollen chamber. The group receiving five preseasonal injections of BM32 showed the best clinical effect, yielding 23.8% lower daily symptom and medication scores during pollen season compared to placebo.
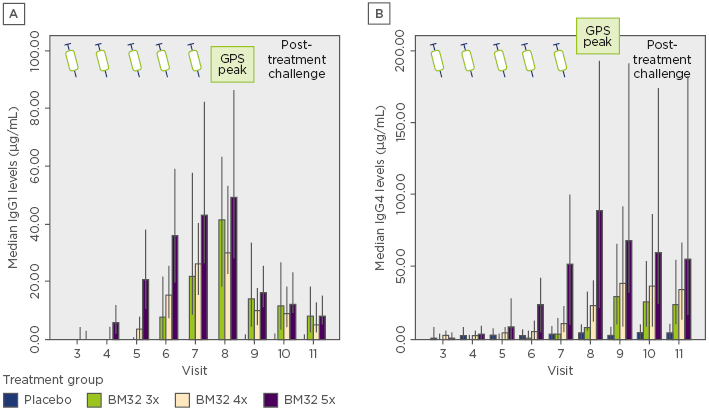
Figure 1: Dose-dependent induction of Phl p 1 and Phl p 5-specific immunoglobulin G1 (A) and immunoglobulin G4 (B) levels.
Error bars indicate the 95% confidence interval range.
GPS: grass pollen season; Ig: immunoglobulin.
BM32 was safe and well tolerated in all clinical studies. Mostly local injection site reactions were recorded by the patients, and very few and only late-onset systemic reactions, mainly Grade ≤2, were described.
In conclusion, BM32 is therefore a valuable candidate for a high-dose, short-course immunotherapy to treat grass pollen-allergic patients effectively and safely.






