Chairperson: Yvan Vandenplas1
Speakers: Carina Venter,2 Norbert Sprenger,3 Anna Nowak-Wegrzyn4
1. KidZ Health Castle, UZ Brussel, Vrije Universiteit Brussel, Brussels, Belgium
2. Section of Allergy and Immunology, Children’s Hospital Colorado and University of Colorado, Denver, Colorado, USA
3. Nestlé Institute of Health Sciences, Société des Produits Nestlé S.A., Lausanne, Switzerland
4. Allergy and Immunology, Department of Pediatrics, Hassenfeld Children’s Hospital, NYU Langone Health, New York City, New York, USA
Disclosure: Dr Venter has provided and reviewed education material for Danone, Abbott, Reckitt Benckiser Group plc, and DBV technologies; provided research support to Reckitt Benckiser Group plc; and received speaker honorarium from Nestlé Health Science S.A. Dr Sprenger is an employee of Société des Produits Nestlé S.A., at the Nestlé Institute of Health Sciences S.A. Prof Nowak-Wegrzyn has received honorarium from Nestlé Health Science S.A. for this presentation as well as a research grant for the clinical study of whey-based extensively hydrolysed formula with two human milk oligosaccharides, for which she was principal investigator.
Acknowledgements: Medical writing assistance was provided by Jennie James, Surrey, UK.
Support: The publication of this article was funded by Nestlé Health Science. The views and opinions expressed are those of the speakers.
Citation: EMJ Allergy Immunol. 2020;5[1]:28-36.
Meeting Summary:
This symposium explored the role of nutritional interventions relating to food allergy, first in terms of prevention strategies and then management, focussing on the benefits of human milk oligosaccharides (HMO) in the management of cow’s milk protein allergy (CMPA). The first speaker, Dr Venter, discussed the benefits of introducing allergenic foods at the time of commencing complementary feeding to prevent future food allergies. She summarised current guidelines on food allergy prevention, which are all in agreement that there is no reason to withhold or delay the introduction of allergenic foods in infancy, and discussed the logistics of food allergen introduction in the first year of life. The talk ended with promising research associating a diverse diet in infancy with reduced risk of food allergy over early and late childhood. Next, Dr Sprenger described how HMO, a major component of breast milk, play an important role in supporting the immune system of infants. He shared evidence suggesting that specific HMO protect breastfed infants from infections as well as clinical findings showing that the addition of two common HMO to regular infant formula helps protect healthy infants from lower respiratory tract infections (LRTI) and related antibiotic use, likely in part through their effects on the gut microbiome of young infants. Prof Nowak-Wegrzyn then reviewed the latest trial data showing that the addition of two HMO to hypoallergenic formula also reduced the risk of respiratory tract infections in infants with CMPA.
How to Implement Nutritional Strategies for Food Allergy Prevention
Doctor Carina Venter
Guidelines for the prevention of food allergy have evolved significantly over the last two decades. The definition of an infant at high risk of food allergy has shifted from being based on a familial risk of allergic diseases to an infant’s own risk of developing food allergy; infants at higher risk include those with eczema and/or an existing food allergy (egg allergy in particular is considered to be a risk factor for the development of peanut allergy). Advice for families to avoid peanut and/or other food allergens during pregnancy, breastfeeding, and the infant’s first few years of life has also been abandoned in light of accumulating evidence that early food allergen introduction can be beneficial for the prevention of future food allergies.1
In the last few years, guidance on when to introduce food allergens into the infant diet has been developed. This followed the publication of a number of studies, in particular the LEAP and EAT studies which suggested or recommended no need to delay active introduction of food allergens, particularly peanut and egg, in the infant’s first year of life. The guidance related to both infants at higher risk and those in the general population.2,3
A summary of current guidelines showed that the practical approach is to introduce food allergens when the infant is developmentally ready to eat and once the infant has consumed a few foods. Dr Venter explained this is irrespective of the infant’s risk of developing food allergies because until it is possible to clearly define ‘high risk’, it is perhaps sensible to treat all infants the same. This practical approach is validated by scientific opinions from both the United States Department of Agriculture (USDA) and the European Food Safety Authority (EFSA), which recommend introduction of solid foods once the infant is developmentally ready.4,5 Both suggest introducing peanut and cooked egg in early infancy for the prevention of peanut and egg allergy, respectively.
There are practical dilemmas for food allergen introduction, such as the appropriate dose to give and the development of suitable food formats for young infants. Randomised controlled trial outcomes provide some guidance on dose, duration, frequency, and format, but currently there is only clear data about peanut introduction and limited data about egg introduction. Guidance published by the National Institute of Allergy and Infectious Diseases (NIAID) recommends about 1–2 teaspoons of an infant-safe form of peanut, such as peanut flour or thinned peanut butter, served 1–3 times a week.6 With regard to egg, it should be cooked according to the EAT and PETIT studies,3,7 but what constitutes a well-cooked egg is debated, although it does include boiled and scrambled egg if it has been heated until no visible liquid remains. The dose recommended for prevention of egg allergy is also debated, but 1–2 tablespoons of well-cooked egg 1–2 times per week is in line with healthy eating guidance.1
In terms of introducing other major allergens for allergy prevention, there is either limited or no data and so current guidance on intake of these allergens comes from healthy eating guidelines. Dr Venter suggested that even when the data is lacking there is probably still no need to delay the introduction of the allergen, which can form part of normal dietary intake according to family practices.
Diet Diversity for Allergy Prevention
There is also promising research on diet diversity for prevention of food allergy outcomes. Diet diversity can be defined as the number of different foods or food groups consumed over a given reference period.8 To understand the role of diet diversity on allergy outcomes, the European Academy of Allergy and Clinical Immunology (EAACI) brought together a task force that performed a systematic search of the literature (the results of which have been published in a EAACI position paper on diet diversity in pregnancy, infancy, and childhood) and found only one study that focussed on the potential association of diet diversity and development of food allergy.9 This showed that children with a more diverse diet during the first year of life had a lower prevalence of doctor-diagnosed food allergy up until 6 years of age.10
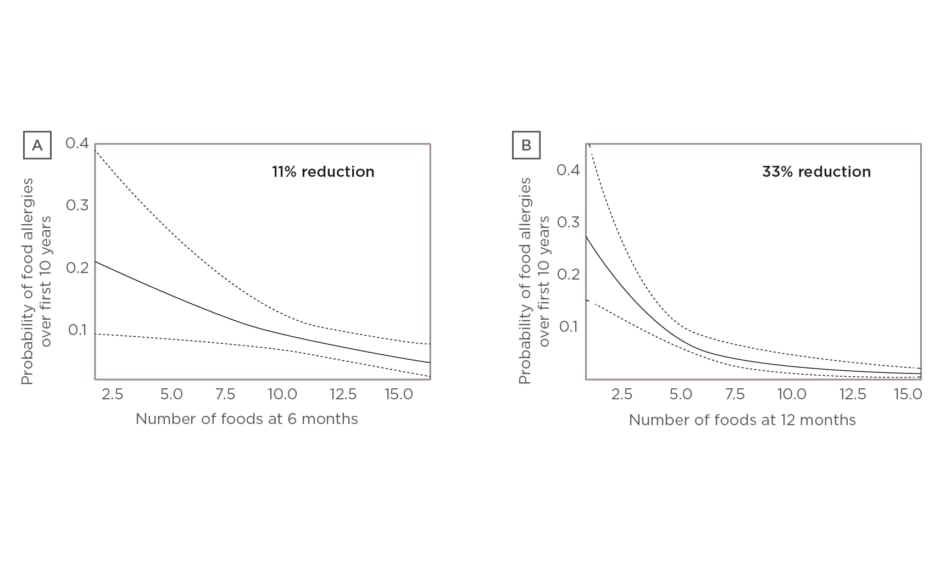
Since then, a new study led by Dr Venter exploring the association between four different measures of diet diversity during infancy and development of food allergy over the first 10 years of life has been published.11 Dr Venter discussed results of two of the measures, focussing on the number of foods introduced over the first 9 months of life and the number of food allergens introduced over the first 12 months. Multivariate analysis showed food diversity at 6 months (p=0.0111) and food allergen diversity at 12 months (p=0.0005) significantly reduced the odds of food allergy over the first 10 years of life (Figure 1A and 1B, respectively).11
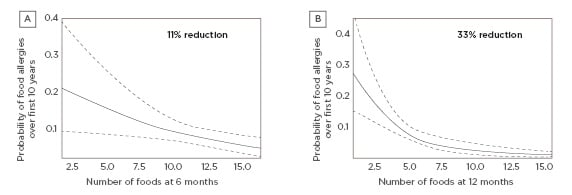
Figure 1: A) Food diversity at 6 months versus food allergy over 10 years. Multivariate analysis showed that food diversity at 6 months (p=0.0111) significantly reduced the odds of food allergy over the first 10 years of life (holding introduction of solids at the mean and ever having eczema: yes).11 B) Food allergen diversity at 12 months versus food allergy over 10 years. Multivariate analysis showed that food allergen diversity at 12 months (p=0.0005) significantly reduced the odds of food allergy over the first 10 years of life (holding introduction of solids at the mean and ever having eczema ever: yes).
Dotted line: 95% confidence interval; solid line: p value.11
The exposure of the gastrointestinal microbiota to diverse foods and nutrients is a plausible mechanism for how diet diversity may affect food allergy outcomes in an infant or child. There are no published data looking directly at how infant diet diversity influences infant microbial diversity. However, increases in family food intake in the first year of life significantly increases the microbial diversity in the infant gut,12 and can potentially be used as a measure of diet diversity. Laursen et el.12 showed that the composition of the complementary diet was a major determinant of gut microbiota development; in particular, the transition to family foods with higher protein and fibre content in the first year of life correlated with increased gut microbiota diversity.
Furthermore, a study by Roduit et al.13 showed that infants with a higher consumption of fish, fruit, vegetables, and yoghurt in the first year of life had increased levels of short-chain fatty acids (SCFA), such as butyrate, in faecal samples. Children with the highest levels of butyrate at 1 year of age were less likely to have a reported diagnosis of food allergy up until 6 years of age.13 SCFA are produced by microbes in the gut and have been shown to have immune regulatory properties in animal studies. As butyrate production increases there is an upregulation of T regulatory cells, which play a key role in sustaining immune tolerance to allergens. According to Dr Venter, these data, together with evidence that increased diet diversity in the first year of life was associated with reduced food allergy outcomes up until 6 years of age, indicate that the microbiome is a plausible mechanism for tolerance induction in infants.
Human Milk Oligosaccharides for Immune System Development
Doctor Norbert Sprenger
Breast milk has a unique composition and contains many immunomodulatory components including HMO, which are the third most abundant solid component after lactose and lipids.14,15
These complex glycans contain a lactose core that is elongated by one or more of the monosaccharides galactose, N-acetylglucosamine, fucose, and sialic acid. HMO are structurally diverse but can be divided into three main classes: core structures, fucosylated HMO (the most common), and sialylated HMO.15 Dr Sprenger explained that structurally, HMO resemble host intestinal epithelial glycans and differ from classical prebiotics, such as galactooligosaccharides and fructooligosaccharides, indicating that they have specific functions that cannot simply be mimicked by other ‘classical’ prebiotics. Two common HMO, 2’-fucosyllactose (2’-FL) and lacto-N-neotetraose (LNnT), have so far been the main focus of basic research and clinical studies into the effects of HMO in infants.
Research suggests HMO support the development of the immune system via several major functions.16-22 These include direct effects, namely preventing pathogen growth and adhesion, reducing inflammatory responses, and aiding the mucosal barrier function, and indirect effects on the microbiota through colonisation resistance and education of the developing immune system.
HMO provide substrates for the developing gut microbiome of young infants, stimulating the growth of bifidobacteria. Several studies associate specific fucosylated HMO with bifidobacteria-dominated early-life microbiota in breastfed infants.23-25 Studies have identified select bifidobacteria strains commonly found in breast milk, including Bifidobacterium longum, which can use HMO as a growth substrate. They do this by either consuming the HMO entirely (internalisation) or nibbling off the end products, such as fucose and sialic acid, which are then either internalised or left to crossfeed other microbes (extracellular breakdown).26-29
Specific HMO, including 2’-FL, not only boost the growth of bifidobacteria, but also the metabolic activity of specific bifidobacteria related to immune protection, primarily as higher formation of the SCFA acetate. This results in improved gut-barrier function and has other broader anti-inflammatory effects.25 HMO also directly reduce microbial infections by serving as antiadhesive antimicrobials. Because HMO resemble mucosal glycans, they can act as decoy receptors for pathogens, such as Campylobacter jejuni, that would otherwise interact with mucosal glycans to invade the host and cause disease.16,17
Specific Human Milk Oligosaccharides Support the Immune System
Clinical observational studies in breastfed infants have associated breast milk containing 2’-FL, and related 2’-FL-HMO, with lower morbidity, respiratory infections, and diarrhoea.30-34 2’-FL-positive breast milk has also been shown to help alleviate the effects of caesarean birth on infant gut microbiota in a study that investigated the differences between gut-microbiota composition in infants with mothers that secrete milk with high 2’-FL content (secretors) and those with mothers who do not (nonsecretors), taking into account birth mode. It found that many of the caesarean-associated dysbiosis patterns were more pronounced among the infants of nonsecretors compared to those of secretors; particularly, bifidobacteria were strongly depleted and enterococci increased amongst this group.35 In the same cohort, these findings relate to the observed reduced risk to manifest IgE-mediated allergic symptoms in caesarean-born infants breastfed by mothers expressing 2’-FL compared to the nonsecretor-fed infants at 2 years of age.36
Benefits of Formula Supplemented with Human Milk Oligosaccharides
Results from a randomised controlled trial showed that feeding healthy infants a formula containing 2’-FL modified their innate and adaptive immune profiles to be similar to that of a breastfed reference group. Similar to the breastfed infants, the infants fed the formula containing 2’-FL had lower inflammatory cytokines compared to those fed a control formula.37
A study by Puccio et al.38 explored the effects of infant formula supplemented with 2’-FL and LNnT on growth and morbidity in healthy infants. In this multicentre trial, healthy, full-term infants who were not breastfed and aged 0–14 days at the time of enrolment were randomised to receive regular infant formula supplemented with 2’-FL and LNnT (n=88) or a control formula (the same formula without the HMO; n=87). The study met its primary endpoint, showing the formula with 2’-FL and LNnT was well tolerated and supported normal growth in healthy infants for a 4-month period. Secondary findings showed that infants fed the two HMO formula had significantly lower rates of parent-reported morbidities related to LRTI, particularly bronchitis, as well as reduced antibiotic and antipyretic use for a 12-month period compared to those fed control formula (Figure 2).38
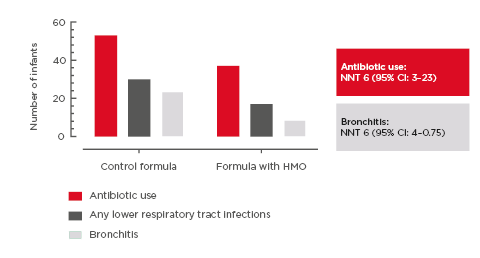
Figure 2: Number of infants with >1 event during first year of life.
Lower morbidity, particularly bronchitis, and antibiotic use were reported in infants fed formula supplemented with 2’-fucosyllactose and lacto-N-neotetraose versus control formula.
NNT: number needed to treat.
Adapted from Puccio et al.38
In the same study, Berger et al.39 then investigated whether HMO-driven microbiota changes relate to the observed reduced risk for reported antibiotic use and LRTI. They compared microbiota composition at 3 months of age across the two randomised formula-fed groups (regular formula supplemented with 2’-FL and LNnT and control formula) and a breastfed reference group. Results showed that the microbiota composition in infants fed HMO-supplemented formula was closer to that of the breastfed reference group than those fed control formula, mainly because of increases in Bifidobacterium concomitant with decreases in Escherichia and Peptostreptococcaceae.39
Furthermore, looking at the association of the microbiota community type with reported morbidities and medication use up to 12 months, formula-fed infants with a community type resembling that of breastfed infants at 3 months were significantly less likely to need antibiotics during the first year than those with the distinct community type typical of control formula-fed infants. This suggests that the reported lower rates of infection-related medication use with HMO may be linked to gut microbiota community types.39 Although the researchers did not observe any significant association between other reported clinical parameters and the 3-month community type, the caesarean-delivered infants showed more differences between formula groups than the vaginally delivered infants, suggesting a stronger normalisation effect of the two HMO on dysbiotic microbiota.39
Further exploration of possible gut microbial mechanisms that relate to the beneficial clinical outcomes in infants fed formula enriched with 2’-FL and LNnT also showed higher levels of acetate per total SCFA and specific Bifidobacterium species at 3 months in the stools of infants who did not experience bronchitis or any LRTI during the first year.40 In summary, Dr Sprenger noted that findings in formula-fed infants suggest the HMO 2’-FL and LNnT help protect from LRTI and antibiotic use, likely in part through their effects on the early-life gut microbiome establishment and function.
New Data on the Benefits of Human Milk Oligosaccharides in the Management of Cow’s Milk Protein Allergy
Professor Anna Nowak-Wegrzyn
Breast milk is the gold standard of infant nutrition. It has the capacity to influence immune-related outcomes in infancy and early childhood and contains many immunomodulatory ingredients including HMO, which provide the substrate for the developing gut microbiome of infants. More than 200 HMO have been detected to date, and a large Finnish study has suggested that the profile of HMO in breast milk may influence development or may be a risk factor for development of CMPA. This study found that the breast milk of mothers whose infant had CMPA had a different composition of HMO compared to mothers of nonallergic infants.41
The two HMO, 2’-FL and LNnT, have been selected for inclusion in the new generation of infant and specialty formulas to more closely emulate the profile of breast milk. Prof Nowak-Wegrzyn reviewed new evidence supporting the addition of HMO as an important component of a hypoallergenic whey-based extensively hydrolysed formula (EHF) for the management of CMPA. The IVORY trial was the first clinical trial of a whey-based EHF containing two HMO in CMPA, designed to evaluate its hypoallergenicity. This multicentre, randomised clinical trial included 67 infants and children aged between 2 months and 4 years with physician-diagnosed IgE-mediated CMPA and who were healthy and born at term. It compared currently marketed EHF without HMO with the newly modified EHF supplemented with 2’-FL and LNnT; this was slightly different in that it had a lower content of whey protein: 2.2 g/per 100 kcal versus 2.5 g/100 kcal in the current EHF. Results showed the new HMO-supplemented EHF met the American Academy of Pediatrics (AAP) criteria for hypoallergenicity, being tolerated by >90% of subjects.42
Benefits of Human Milk Oligosaccharides Extend to Infants with Cow’s Milk Protein Allergy
Research suggests that HMO protect against infection, both in breastfed and healthy infants fed formula supplemented with two HMO. Infants exclusively breastfed for at least 4–6 months had a lower incidence of upper respiratory tract infection (URTI), LRTI, and gastrointestinal infections compared to fully formula-fed infants.43 This is thought to be related to the immune-modulating and microbiome-modifying effects of HMO.44 Healthy infants fed a regular formula supplemented with 2’-FL and LNnT since birth had significantly fewer LTRI and required significantly fewer courses of antibiotics in the first year of life compared to those fed a non-HMO containing formula.38
Therefore, the next step was to evaluate whether similar benefits would be seen with a hypoallergenic whey-based EHF supplemented with 2’-FL and LNnT in infants with CMPA. Prof Nowak-Wegrzyn described how infants with CMPA and food allergies in general are more susceptible to infections because of the immaturity of their immune system.
The CINNAMON trial45 was designed to assess whether the newly modified EHF supplemented with 2’-FL and LNnT supports normal growth (primary endpoint) and whether it has beneficial effects on infection rates and related drug use in infants with CMPA.
This double-blind, randomised, multicentre, interventional study compared the same two formulas used in the IVORY study and included older infants (aged 0–6 months at enrolment) compared to the Puccio study38 of the regular formula supplemented with 2’-FL and LNnT in healthy infants. The study met its primary endpoint, with the two groups showing comparable weight gain per day at 4 months (19.38 g/day on test versus 20.12 g/day on control formula).45
Prof Nowak-Wegrzyn discussed the secondary outcomes, specifically infection rate, noting that the study replicated many of the observations from the Puccio study38 in healthy infants. The full analysis set (N=190) showed that the fraction of infants with at least one LRTI from enrolment to 12 months of age was reduced by 33.6% (odds ratio [OR]: 0.61; p=0.25), and URTI episodes were reduced by 5.2% (OR: 0.91; p=0.77) for HMO-supplemented EHF versus control EHF.46 While the calculated OR were generally in favour of the HMO-supplemented EHF, the confidence intervals were such that those differences were not statistically significant. According to Prof Nowak-Wegrzyn, this may, in part, be because the study may not have been powered to detect these secondary outcomes. Another confounder was the older age at the introduction of the HMO-supplemented EHF in infants with CMPA: the average age was 3.2 months (standard deviation: 1.7) compared with healthy infants fed with the HMO-supplemented formula since birth. The earlier introduction of the HMO-supplemented formula might have a more favourable impact on establishment of a healthy gut microbiota and modulation of infection risk. Risk of otitis media was also reduced by 70–100%, with a significant difference between the HMO-supplemented and control EHF on per protocol analysis (n=147; OR: 0.00–0.44; p<0.05).46
Specifically, there was a significant reduction of 42% (p=0.003) in the frequency of URTI episodes to 12 months of age in infants fed the HMO-supplemented EHF compared to those fed control EHF (Figure 3); the frequency of LRTI was reduced by 23% (p=0.61).46
Figure 3: Incidence rate of respiratory tract infections per month of exposure to the whey-based extensively hydrolysed formula supplemented with 2’-fucosyllactose and lacto-N-neotetraose versus extensively hydrolysed formula without HMO (control). Full analysis set (from enrolment to 12 months).46
Error bars indicate 95% confidence interval.
HMO: human milk oligosaccharides.
Summarising, Prof Nowak-Wegrzyn said it was interesting that these reductions in frequency of infections in CMPA were consistent with reduced respiratory infections amongst nonallergic, healthy infants fed regular formula supplemented with 2’-FL and LNnT since birth. She further stated that these findings support and advance the concept of modifying infant formula to enrich it with the immunomodulatory components that are present in breast milk.