Introduction
Domestic cats, Felis catus, are one of the major sources of indoor allergens responsible for various allergies, including respiratory disorders.1 There has been a steady rise in the prevalence of sensitisation to cat allergens,1 which may be manifested as atopic symptoms in a substantial proportion of allergic individuals.2,3 Cat ownership is fairly common in Western countries; almost a quarter of households in the USA4 and Europe5 own a pet cat, and it can be reasonably assumed that some of these households will have individuals who are allergic to cats. Consequently, management of cat allergens poses a substantial burden on these individuals.6,7
Cats produce a number of potential allergens. To date, ten allergens, recognisable by their interaction with specific IgE, have been identified in extracts derived from feline hair, saliva, serum, and urine.8,9 Moreover, eight of these allergens, Fel d1–8, have been registered with the World Health Organization (WHO) and International Union of Immunological Societies (IUIS).1 Of these, Fel d1 is the most important allergen to which most individuals with cat allergies are sensitised.10,11 Fel d1 is a 35–39 kDa glycoprotein produced primarily by the salivary and sebaceous glands in cats.12 While the exact biological role of Fel d1 is yet to be determined, it is proposed to have numerous functions such as skin protection or transport of lipids, especially steroids, hormones, and pheromones.1 Fel d1 is transferred to the hair when cats groom and is subsequently shed with hair and dander. Owing to its small size and molecular structure, Fel d1 can be airborne for long periods of time as well as adhere to fabrics and indoor furniture,13,14 thereby increasing the probability of exposure. On exposure in sensitised individuals, the unbound form of Fel d1, termed active Fel d1 (aFel d1) in this report, binds IgE and leads to mast cell degranulation, thus initiating the allergic response cascade.
In most cases, treatment approaches for cat allergies are palliative and consist of anti-allergy medications (e.g., antihistamines or decongestants) or avoiding exposure through restriction of access, physical removal of the cat, and improvement of air quality using filtration units. Another line of treatment is allergen immunotherapy, which in principle involves exposure to increasing amounts of allergens to desensitise the individual and induce immune tolerance.15 While this approach has had success for other allergens, there is little data to support allergen immunotherapy for regular use against cat allergies.16 Moreover, the process is long-term, contraindicated in conditions such as asthma, and cost-restrictive.17
The authors’ approach to managing cat allergens takes advantage of the antibody-antigen interaction by generating anti-Fel d1 antibodies using the avian IgY system. The avian IgY is equivalent to the mammalian IgG and is naturally produced in domestic birds, such as chickens, in response to antigens. These antibodies are transferred to the egg where they provide passive immunity to the hatchlings. These antigen-specific IgY antibodies subsequently attach to targeted antigens and neutralise or mark them for destruction by cells of the immune system. The use of avian IgY is not a new discovery: it has already been in use for numerous animal and human applications.18,19 However, the element of novelty in the authors’ approach is that instead of administering the IgY antibodies to the human patient, this research focusses on incorporating the anti-Fel d1 IgY antibodies in the cat’s diet, through a safe and nutritious egg product, with the intention of neutralising the aFel d1 in cat saliva. While it was not expected that such an approach would alter the amount of Fel d1 secreted, it was hypothesised that it would reduce the amount of aFel d1 transferred to the hair during grooming and dispersed into the cat’s environment.
In this article, the speakers review and share some of the data presented in four posters at the European Academy of Allergy and Clinical Immunology (EAACI) Congress held from the 1st–5th June 2019 in Lisbon, Portugal.
Variability in Fel d1 Levels in Cat Saliva
There are many misconceptions and conflicting reports related to cat allergens,20 including the propensity of specific cat breeds to secrete lower levels of Fel d1, and hair colour or length and gender being associated with Fel d1 levels.21-24 Hence, one of the first questions Bastien et al.25 sought to answer in the study presented in this poster was whether specific factors such as age, weight, and breed had any effect on the levels of Fel d1 secreted in feline saliva.
In this study, the authors recorded data from 64 healthy, neutered cats of either gender, aged 1.2–15.3 years, and varying in breed, weight, hair colour, and pattern. The principal measurement was salivary levels of Fel d1 in samples collected twice a day, pre and post-feeding, every other day for a year. Fel d1 levels were measured using a commercially available Fel d1 ELISA kit (Indoor Biotechnologies, USA).
It was observed that there was no association between salivary Fel d1 levels and factors such as bodyweight, body condition score, and the colour and pattern of the hair. However, it was interesting to note that Fel d1 production varied substantially not only between cats, but within the same cat over the course of a year. In addition, the age of the animal was found to influence salivary Fel d1 levels; older animals (11–15 years) had significantly lower salivary levels of Fel d1 in comparison to younger animals (1–5 years; p<0.001). There was an 80-fold difference in Fel d1 levels between the highest and lowest Fel d1-producing cats. Moreover, cats with a lower average Fel d1 had lower variability over the year compared to cats with higher average Fel d1 levels. These results indicate that salivary samples taken at a single point are not ideal for estimation of the cat’s average Fel d1 level and biological variability of Fel d1 should be taken into account in future studies. The methodology and results of these studies have recently been published in a peer-reviewed article.26
Fel d1 Blocking Antibodies Against the Major Cat Allergen Fel d1
To test whether anti-Fel d1 antibodies have the potential to block feline Fel d1 and downstream degranulation of mast cells, Satyaraj et al.27 conducted a series of mechanistic studies using a chimeric ELISA and the beta-hexosaminidase release assay, respectively. The authors first tested the specificity of the antigen-antibody binding using various dilutions of two distinct rabbit-derived anti-Fel d1 antibodies: the Indoor Poly (Indoor Biotechnologies Inc., USA) polyclonal antibody, which recognises multiple Fel d1 epitopes; and Fel d1 Major allergen 1 polypeptide chain 1 antibody (FGI; Fabgennix International Inc., USA), a monoclonal antibody that identifies the peptide sequence covering amino acids 23–40 in chain 1 of Fel d1, a known IgE-binding site.28 The authors discovered a dose-dependent inhibition of salivary Fel d1 and human IgE complex formation with the Indoor Poly antibody (p<0.001) but none with the monoclonal FGI antibody (unpublished results). This indicates that multiple epitopes on Fel d1 need to be blocked to prevent its interaction with IgE and the subsequent mast cell degranulation. It was next investigated if either anti-Fel d1 antibodies could attenuate the Fel d1-mediated activation of IgE and the subsequent allergic cascade, measured through the release of beta-hexosaminidase in humanised rat basophilic leukaemia cells. A specific and dose-dependent reduction in beta-hexosaminidase release was discovered when the polyclonal, but not monoclonal, anti-Fel d1 antibodies were incubated with cat saliva samples.
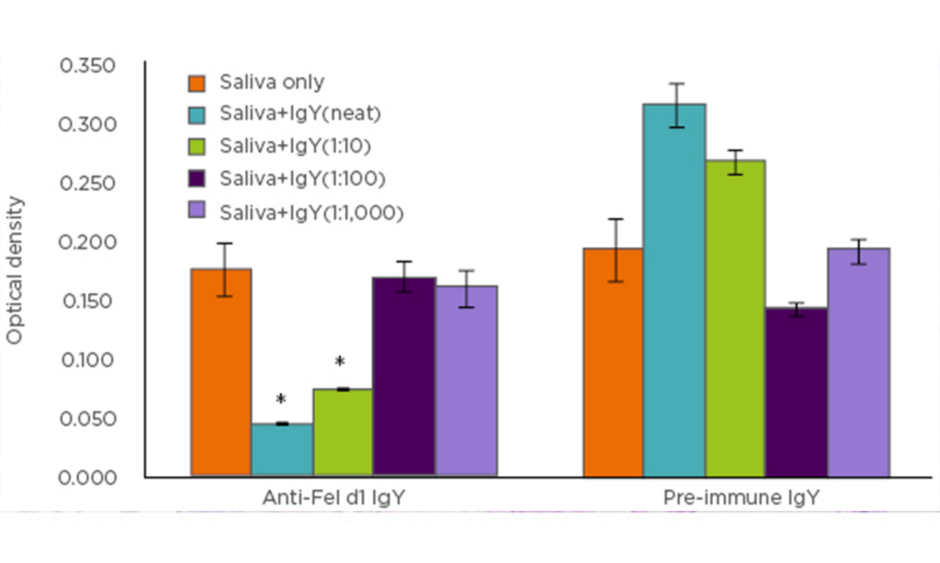
The authors next sought to examine whether these observations could be replicated using anti-Fel d1 IgY antibodies isolated from egg yolks instead of the polyclonal anti-Fel d1 antibodies. Combining the results of the chimeric ELISA (Figure 1), as well as beta-hexosaminidase assay, a specific and dose-dependent reduction in Fel d1 binding to IgE and downstream mast cell activation was discovered.
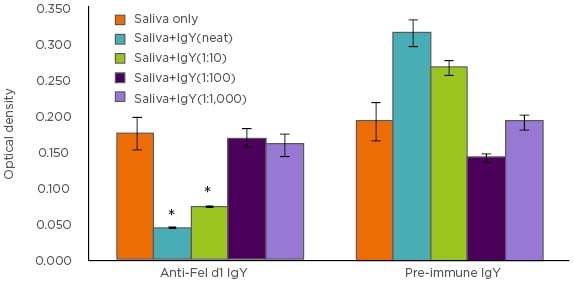
Figure 1: Chimeric ELISA comparison of IgY antibodies isolated from eggs produced by Fel d1-immunised (anti-Fel d1 IgY) hens compared to IgY from the same hens prior to immunisation (pre-immune IgY).
*p<0.001
The authors next aimed to ascertain if these in vitro results could be translated in an animal model. To answer this question, a pilot feeding trial was conducted in 20 cats with moderate-to-high, yet reasonably stable, salivary Fel d1 concentrations. Following a control diet period of 1 week, the cats were split into two groups: one to continue with the control diet, and the other to receive the control diet supplemented with an egg product ingredient containing anti-Fel d1 IgY antibodies, for 4 weeks. Saliva samples were collected weekly and Fel d1 levels therein were estimated using commercially available direct ELISA (Indoor Biotechnologies, USA). It was discovered that, by Week 3, cats receiving the ingredient containing anti-Fel d1 IgY in their diet had an approximately 24% reduction in their salivary aFel d1 levels in comparison to baseline.
With these data, it can be concluded that Fel d1-specific IgY antibodies bind Fel d1 in cat saliva, thereby blocking the ability of the latter to bind IgE and induce IgE-mediated degranulation. Since monoclonal anti-Fel d1 antibodies could not produce the same effect, it can be concluded that multiple epitopes of Fel d1 must be blocked to prevent its binding to IgE and initiation of the IgE-mediated allergic response cascade. Perhaps the most interesting observation made was the reduction in salivary aFel d1 levels in cats fed a diet with an egg product ingredient containing anti-Fel d1 IgY, which then offers a novel strategy to neutralise aFel d1 in cat saliva without altering production.
Effects of Fel d1 Blocking Antibodies on Levels of Fel d1 on Cat Hair
After conclusively demonstrating that anti-Fel d1 IgY dose-dependently blocked salivary Fel d1 in vitro and reduced salivary levels of aFel d1 in cats whose diet was supplemented with anti-Fel d1 IgY antibodies, Satyaraj et al.29 next sought to ascertain whether this effect could be replicated on the levels of aFel d1 on hair in cats fed a diet with an egg product ingredient containing the anti-Fel d1 IgY antibodies.
Thus, 105 domestic shorthair cats were enrolled, aged 7 months to 17 years, of either gender, and neutered or intact, in a 2-week baseline period on a control diet followed by separation into two groups. Cats in the control group continued to receive the same diet for a further 10 weeks while cats in the test group were fed the control diet supplemented with an egg product ingredient containing anti-Fel d1 IgY for 10 weeks. Hair samples were collected from the most commonly groomed areas (belly, shoulders, and sides) twice weekly during the baseline period and once per week during the 10-week study phase. Approximately 100 mg of each sample was quantitatively analysed for aFel d1 levels using the previously mentioned ELISA kit.
It was observed that by the end of the study period 97% of the test group cats showed reduction of aFel d1 levels on hair compared to baseline; in 86% of the cats the reduction was ≥30%. Substantial reductions in aFel d1 levels were observed from Week 3 onwards (p<0.001), and on average the reduction in aFel d1 levels was 47% by Week 10 (Figure 2A). Thus, it can be concluded that feeding cats a diet with an egg product containing anti-Fel d1 IgY resulted in reduction of aFel d1 levels in the hair and dander and the greatest decreases were observed in cats with initially high levels of Fel d1 (Figure 2B). These results reinforce the previous findings on a larger scale and establish the proof-of-concept of a novel approach for managing cat allergies. The details and results of this study were recently published in a peer-reviewed journal.12
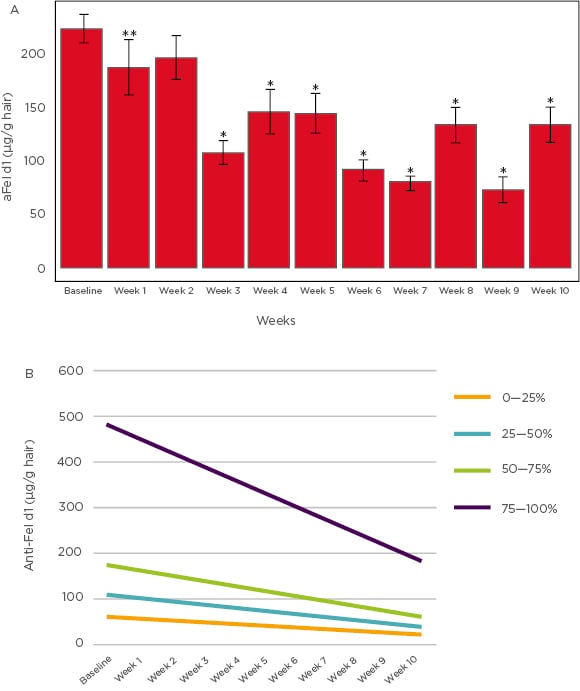
Figure 2: A) Mean active Fel d1 levels (µg/g hair) across 10 weeks. B) Mean change in Fel d1 levels based on initial concentrations.
*p<0.001 **p<0.050
Pilot Evaluation of Cats Fed Anti-Fel d1 Antibodies and Human Allergy
Having demonstrated a blockade of Fel d1 by anti-Fel d1 IgY in vitro and in vivo accompanied by subsequent reduction in aFel d1 secreted in feline saliva and hair, Wedner et al.30 intended to investigate if this resulted in beneficial effects in humans with cat allergies. To do this, a pilot study was conducted in which human volunteers were exposed to bedding used by cats fed either a control diet or a test diet with an egg product ingredient containing anti-Fel d1 IgY.
Briefly, blankets on which cats (fed on one of these two diets) had laid and shed hair were used as source of Fel d1 in environmental chambers (FlowerHouse Inc., USA) set up to provide controlled exposure. Eleven adult human subjects with a history of cat sensitivity confirmed by a positive skin prick test to cat allergens and a documented variable response to high and low Fel d1 levels were recruited to participate in this 4-week randomised, double-blinded, crossover study. Subjects underwent a priming exposure during Week 1, in which they spent 3 hours in the environmental chambers containing blankets from cats fed the control diet. Following this, subjects underwent two more exposures during Week 2 and Week 4 to hair from cats that had been fed either the control diet or the test diet. Subjects randomly assigned to control exposure in Week 2 were exposed to the test condition in Week 4 and vice versa, thus each subject served as their own control. During the exposure period, subjects filled in questionnaires assessing Total Nasal Symptom Score (TNSS) and Total Ocular Symptom Score (TOSS) every 15 minutes. The control and test exposures were compared to the initial priming exposure for statistical evaluation to reduce potentially confounding placebo effects.
It was noted that the levels of aFel d1 were lower in chambers loaded with blankets from cats fed the test diet in comparison to those with blankets from cats fed the control diet. Overall TNSS score and the subscore for nasal congestion were substantially reduced in subjects exposed to hair from cats fed the test diet in comparison to the priming exposure (p=0.0350 and p=0.0055, respectively). The authors also observed improvement in other allergic symptoms such as nasal itching, sneezing, and runny nose; however, the reduction in these scores was not statistically significant. While reduction in overall TOSS scores did not reach statistical significance, subscores for scratchy eyes and itchy eyes were significantly decreased (p=0.0150 and p=0.0072, respectively).
With this study, it was demonstrated that humans with feline allergies had substantial improvement in their TNSS scores and some ocular scores when exposed to bedding from cats fed a diet with an egg product ingredient containing anti-Fel d1 IgY as compared to bedding from control diet-fed cats. While these results are encouraging and hold the promise of reducing incidence of allergies in cat-owners sensitive to cat allergens, specifically Fel d1, this needs to be replicated in a larger human population.
Summary and Conclusions
While the typical approaches to managing feline allergies have involved either desensitising cat-allergic patients or mitigating the allergic symptoms, this approach was unique in the sense that the authors aimed at neutralising the major cat allergen, Fel d1, after its production but before human exposure, by incorporating an egg product ingredient containing anti-Fel d1 IgY antibodies into the cat’s diet. In our series of studies, the mechanism by which anti-Fel d1 IgY bound feline salivary Fel d1 and prevented it from binding human IgE was demonstrated, thereby curtailing the subsequent mast cell-mediated allergic response. In addition, it was shown that cats fed a diet with an egg product ingredient containing anti-Fel d1 IgY have lower aFel d1 levels in their saliva and hair. However, the most clinically relevant of these data showed an improvement in nasal and some ocular symptoms in individuals sensitised to Fel d1 with this approach. This set of results offers proof-of-concept of a novel and cutting-edge approach to management of allergies in individuals sensitised to Fel d1. Further research will be required to demonstrate the usefulness of this approach for managing cat allergens in the home.