Abstract
Introduction: First-line regular systemic treatment for atopic dermatitis (AD) in the UK consists of methotrexate, azathioprine, ciclosporin, or mycophenolate (immune-suppressive therapies [IST]). ISTs have been associated with malignancy, hence the need for evaluation for the relationship to the risk of developing cancer.
Method: This retrospective cohort study utilising the Clinical Practice Research Datalink (CPRD) followed two cohorts with moderate or severe AD: one prescribed ISTs and one without. A total of 222,978 patients were included. The index date was the date of first IST prescription within primary care for the IST cohort, and the date of first potent topical steroid prescription from January 2001 to May 2021. Cohorts were propensity matched 1:1, resulting in 17,556 patients per cohort. Cox proportional hazard models were used to model the hazard of a cancer diagnosis. A secondary analysis was carried out on a restricted population, excluding patients with other comorbidities where ISTs were commonly prescribed. A further analysis explored the relation between the dose and the association with the risk of cancer.
Results: Both the primary (hazard ratio: 1.01; 95% confidence interval: 0.94–1.08) and secondary (hazard ratio: 1.03; 95% confidence interval: 0.93–1.14) analyses did not show a significant difference in the hazard of a cancer code in the IST and non-IST cohorts. The exploratory dose–response analysis showed a higher risk of cancer associated with more prescriptions of IST per year.
Conclusion: This study shows that amongst patients with moderate or severe AD, overall IST prescription in primary care is not associated with the onset of a cancer code. However, there is a trend with a higher risk of cancer coding with more prescriptions of IST.
INTRODUCTION
Atopic dermatitis (AD) is the most common inflammatory skin condition, affecting 11–20% of children and 5–10% of adults.1 The pathophysiology of AD involves both skin barrier defects and immune dysregulation.2 The universal initial treatment for AD includes topical treatments, such as emollient to support the skin barrier, and topical corticosteroids to manage the immune dysregulation.3 Common further treatment in primary care includes the prescription of more potent topical corticosteroids or intermittent and short-term use of systemic corticosteroids.4 Upon referral to secondary care, patients can be treated with topical calcineurin inhibitors, ultraviolet light therapy, and systemic immune-suppressive therapy (IST) before consideration for biologic or JAK inhibitor therapy. Current further management includes potent topical corticosteroids; systemic steroids; tacrolimus; systemic IST, such as methotrexate, azathioprine, ciclosporin, and mycophenolate; biologics; and JAK inhibitors. The majority of the diagnoses of AD in the UK are carried out by a general practitioner in primary care.5 Severity of AD can be assessed through various methods, including patient-orientated scoring of AD (PO-SCORAD), patient-orientated eczema measure (POEM), and patient global assessment (PGA). Depending on the patient reported outcome measure, approximately 53–68% of patients have moderate-to-severe AD.5 Although there is no consensus definition for moderate and severe AD, within database and claims research, potent and very potent topical corticosteroids, systemic corticosteroids, and IST have been used as surrogates.6
The use of systemic immune modifiers is associated with numerous adverse events, and requires close monitoring. Ciclosporin is associated with nephrotoxicity, hypertension, infection, hypertrichosis, headache, and malignancy.7 Methotrexate is associated with bone marrow suppression, pulmonary fibrosis, skin cancer, and lymphoma.8 Azathioprine has been associated with an increased risk of bone marrow suppression, infection, lymphoma, and non-melanoma skin cancer development.9 Mycophenolate is associated with infection, gastrointestinal events, and lymphoma.10
Prior studies have shown inconsistent results with respect to the background risk of AD. A 50% increased risk of any cancer was observed in patients with AD compared with controls.11 In two large cohort studies in England and Wales, no evidence was found of an increased risk of most cancers among people with AD compared to those without AD. However, within this study, there was an increased risk of lymphoma, with risk increasing with greater severity.12 This result is partially explained by a statement in the American Academy of Dermatology (AAD) guidelines: “An increased risk of skin cancer and lymphoma may be observed with use of immunosuppressive drugs in AD.”13 An increased risk for cutaneous melanoma was found in patients treated with methotrexate for their psoriasis.14 A follow-up study in this same population found no dose–response association with cancer risk among users of methotrexate.15 A systematic review of randomised clinical trials for moderate-to-severe AD up to February 2020 identified three trials featuring 140 patients evaluating azathioprine; three trials with 179 patients evaluating methotrexate; and 19 trials featuring 820 patients evaluating ciclosporin.16 Follow-up varied from 12 weeks to 5 years for azathioprine, identifying myelosuppression as an adverse event. Follow-up for trials evaluating ciclosporin varied between 6–52, with nephrotoxicity reported. Follow-up for trials evaluating methotrexate varied from 12 weeks to 5 years. Malignancy did not feature as an adverse event in these studies. A 5-year follow-up study featuring patients prescribed azathioprine and methotrexate included 35 patients, of which only 27 completed the 5-year follow-up. One malignancy featured in each of the treatment cohorts, which was considered not statistically significant.17 The small numbers of patients and short follow-up in these studies make drawing associations of malignancy risk difficult.
In UK primary care, IST prescription for patients with AD is initiated in secondary care by dermatologists, and followed up via a shared care protocol where the primary care practitioner provides prescriptions and blood monitoring. Although the initial prescriptions to titrate the IST dose are provided by the dermatologist, the follow-ups provided by the primary care prescription are recorded within the Clinical Practice Research Datalink (CPRD).18 The accuracy of recording cancer diagnoses in CPRD was compared with hospital episode statistics, with a concordance of 94%.19 Given the number of patients within primary care treated with ISTs, there was opportunity to study the association of IST prescription in primary care with the risk of cancer; this was undertaken utilising the CPRD database.
METHODS
Study Design
This was an historical matched cohort study encompassing a baseline period prior to the index date for the characterisation of patients for matching, and an outcome period to identify time to cancer outcomes. Only cancer codes appearing outside this window were considered, so as to disregard cancer that may be investigated at the consultation at index period.
The index date was defined in the IST group as the date of the first prescription of an IST, or in the control group as the date of the first prescription of a potent topical therapy (potent topical corticosteroid, very potent corticosteroid, or tacrolimus), or oral corticosteroid. A substudy was carried out, excluding patients with baseline comorbidities where ISTs were prescribed. The study adopted a per protocol approach; thus, patients who were prescribed an IST after prescription of a potent topical therapy entered the control cohort at time of topical therapy, and were censored on the date of their first IST therapy.
Data Source
The authors’ retrospective cohort study used CPRD Aurum,18 an ongoing primary care database of anonymised medical records from general practitioners, which is comprised of over 40 million research-acceptable (permanently registered with sufficient data quality) records.18,20 The database is broadly representative of the general population in England in terms of age, sex, and ethnicity, and covers over 20% of the UK population. The CPRD primary care database is, therefore, a rich source of health data for research, including data on demographics, symptoms, tests, diagnoses, therapies, health-related behaviours, and referrals to secondary care. Each year, CPRD must obtain Section 251 regulatory support through the Health Research Authority Confidentiality Advisory Group (HRA CAG). All requests from researchers to gain access to linked data must be approved via the CPRD Research Data Governance (RDG) Process.
Exposure Studied
IST was a composite of prescriptions for methotrexate, ciclosporin, azathioprine, and mycophenolate.
Methotrexate is an antimetabolite most commonly used in chemotherapy and as an immunosuppressant in autoimmune diseases.21
Azathioprine is a medication used in the management and treatment of active rheumatoid arthritis and the prevention of kidney transplant rejection.22
Ciclosporin is an immunosuppressive agent used to treat organ rejection post-transplant. It also has use in certain other autoimmune diseases; treatment of organ rejection in kidney, liver, and heart allogeneic transplants; and rheumatoid arthritis when the condition has not adequately responded to other drugs.23
Mycophenolate mofetil is an antimetabolite and potent immunosuppressive agent used as adjunctive therapy in prevention of allograft rejection, and in the treatment of serious autoimmune diseases.24
All dosages and routes of ciclosporin, methotrexate, azathioprine, and mycophenolate medication documented within primary care records were included in this study.
Inclusion Criteria
Patients included had a diagnostic code for AD or eczema. Codes for nummular, discoid, contact, dyshidrotic, and varicose eczema were excluded. Patients with no relevant AD diagnostic codes ≥15 years old were removed, as childhood AD is frequent and can resolve in adulthood. Patients with documented allotransplant of bone marrow or liver, renal, pancreas, lung, or heart transplant, or metastatic cancer prior to the index date were excluded, as these conditions are frequently treated with ISTs. Alternative subanalysis was carried out excluding patients with a documented condition at or prior to the index date (rheumatoid arthritis, myasthenia gravis, systemic lupus erythematosus, psoriasis, ankylosing spondylitis, and multiple sclerosis) where ISTs may be prescribed.
OUTCOME ASSESSMENTS
The primary outcome of this study was the time to coding of malignant cancer.
Matching and Weighting
Patients prescribed an IST were propensity score matched 1:1 with no replacement for patients without an IST prescription.
Initially, a characterisation of all baseline demographics, comorbidities, and patient variables was carried out for each cohort. The difference between treatment groups was quantified using a p-value of a hypothesis test of difference using a Wilcoxon Rank Sum Test or a Pearson’s Chi-squared test. Additionally, the standardised mean difference was calculated. Variables with missing data were encoded into categorical variables, with a category for the missing value, enabling matching to be performed.
As the treatment pathway is typically the use of a potent topical therapy prior to IST prescription, there was a necessity to match by age at the index date. Other variables that were matched included inflammatory bowel disease,25 BMI,26 smoking status,27 rheumatoid arthritis,28 sex,29 diabetes,30 gastritis,31 chronic liver disease,32 tacrolimus prescription, index year, chronic renal disease,33 and gastro-oesophageal reflux disease.34 Tacrolimus was also included as a matching criterion despite recent evidence that it was not associated with cancer due to historical warnings around its carcinogenic potential.35,36 The age of the first AD consultation was also determined, so that patients who had AD codes as a child which subsequently resolved could be excluded. Matched characteristics are shown in Table 1.
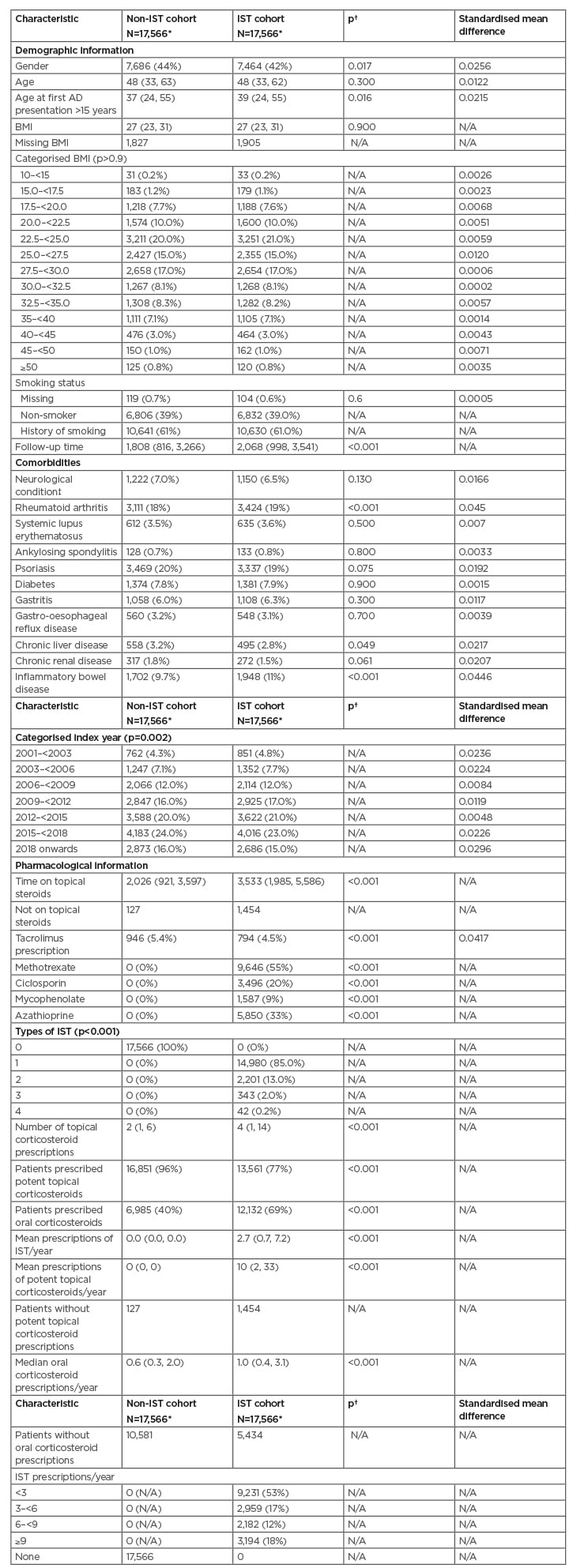
Table 1: Matched characteristics of patients for primary analysis.
*n (%); range; median (IQR).
†Pearson’s Chi-squared test; Wilcoxon Rank Sum Test; Fisher’s exact test.
‡Includes myasthenia gravis and multiple sclerosis.
AD: atopic dermatitis; IQR: interquartile range; IST: immune suppressive therapies; N/A not applicable.
An exploratory analysis for patients with different prescriptions per year post-initial prescription was also carried out, with the comparator being patients with no prescription of IST to establish a dose relationship. The number of prescriptions per follow-up year including index date were categorised into <3 prescriptions/year, 3–<6/year, 6–<9/year, and ≥9/year.
STATISTICAL ANALYSIS
In determining a significant margin to define excess risk of cancer, the authors referred to Mansfield (2020).12 It is stated that a hazard ratio (HR) for cancer of 1.04 (99% confidence interval [CI]: 1.02–1.04) was not appreciably significant, whereas the HR for non-Hodgkin lymphoma was significant at 1.19 (99% CI: 1.07–1.34). Based on this, the authors have assumed that a margin of 10% is clinically relevant. Equal cohort sizes and an α value of 0.05 provided 80% power to detect a difference of 10%, and this required 3,456 patients in each group.
Wilcoxon Rank Sum Test or a Pearson’s Chi-squared test was calculated to identify differences between characteristics during the baseline period. Variables demonstrating a >0.05 standardised mean difference were considered for matching. The propensity score was generated using a generalised logistic model. The calliper used for the propensity score was 0.25 of its standard deviation. For the outcome, 10 matching runs with different random patient orders were made to select the best combination of matched patients and balance statistics.
The intention was to adjust for variables with residual confounding post-matching if the standardised mean difference >0.05, and for cumulative prescriptions for topical corticosteroids and oral corticosteroids.
Conditional proportional hazards regression compared IST to no IST for time to the presence of a cancer code.
During the authors’ preliminary exploration of the data, it was noted that psoriasis and AD were frequently coded in the same patient. Although the prevalence ranged from 0.3% to 12.6%, the pooled prevalence was 2.0%, which was considerably lower than their initial findings. Hence, a secondary study without patients with a history of conditions where IST prescriptions may feature, such as psoriasis, was carried out.
All statistical analyses were performed using R Statistical Software (version 4.1.1; R Foundation for Statistical Computing, Vienna, Austria).
PATIENT DEMOGRAPHICS
After applying the inclusion and exclusion criteria, 17,566 patients prescribed ISTs and 222,978 patients not prescribed ISTs were identified. The authors noted that 6% of patients in the non-IST cohort and 19% of patients in the IST cohort had a diagnostic code for psoriasis.
In the unmatched IST cohort, approximately 55% had been prescribed methotrexate, 20% had been prescribed ciclosporin, 33% were prescribed azathioprine, and 9% had been prescribed mycophenolate. The authors noted that 15% of patients had been prescribed more than one type of IST.
Following matching, the study population consisted of 17,566 patients in each cohort. The median ages of the matched treatment groups were 48 years and 49 years in the IST and non-IST cohorts, respectively.
Age, BMI, categorised BMI, smoking status, and categorised age were balanced. Matched characteristics of these patients are presented in Table 1. Standardised mean difference was lower than 0.05 across all covariates used within the matching, indicating sufficient balance. Forty-two percent of patients were male in the IST compared with 44% in the non-IST cohort. Patients with rheumatoid arthritis were higher in the IST cohort (19% versus 18%). Similarly, there was a higher proportion of patients with inflammatory bowel disease in the IST group (11.0% IST versus 9.7% non-IST). Fewer patients in the IST group had a code for tacrolimus prescription (4.5% versus 5.4%).
In the matched group, median follow-up was 5.7 years in the IST group, and 5.0 years in the non-IST group.
The exploratory analysis cohort of patients without psoriasis, inflammatory bowel disease, rheumatoid arthritis, myasthenia gravis, and multiple sclerosis consisted of 8,175 patients in each of the IST cohort and non-IST cohort.
For the additional analysis of patients with different mean prescriptions per year of IST, matched comparisons were made between patients with <3 prescriptions/year, 3–<6 prescriptions/year, 6–9 prescriptions/year, and ≥9 prescriptions/year, compared with patients with no prescriptions of IST.
PRIMARY ANALYSIS
Patients initiating IST therapy versus patients without IST therapy both had a rate of 2.1 cancers per 100 patient years.
Results of the Cox proportional hazards model indicated that the risk within patients prescribed IST were similarly likely to develop cancer, compared with the patients without a prescription of IST. After adjusting for cumulative potent steroids and oral corticosteroid prescription, the HR was 1.03 (95% CI: 0.93–1.14; p=0.56).
Exploratory Analysis
In the exploratory analysis, patients initiating IST therapy versus patients without IST therapy both had a rate of 1.8 cancers per 100 patient years.
The effect sizes in the additional analyses, where patients with conditions where ISTs are commonly prescribed were excluded, showed that there was no evidence that the risk of cancer in patients with ISTs was higher than in patients with no IST prescription, after adjustment for cumulative potent steroid and oral corticosteroid prescription. The adjusted HR was 1.01 (95% CI: 0.94–1.08; p=0.85).
Additional Dose–Response Analysis
For the additional analysis, a trend of increasing HR was seen for both the primary and exploratory populations with an increasing category of IST prescriptions/year, as illustrated in Figures 1 and 2. The distributions of the bins containing patients with a set number of prescriptions/year was chosen after visualisation of the distribution among the patients. Given that comparing predefined bins may result in selection bias, a logistic regression was carried out for the occurrence of a cancer code against the number of IST prescriptions/year, showing a unit increased risk of 0.103 per prescription/year.
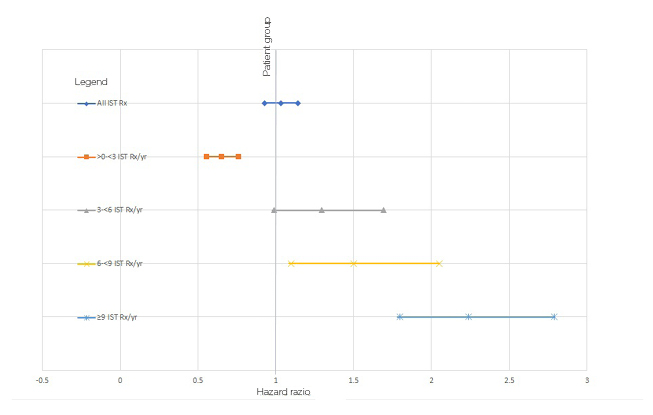
Figure 1: Hazard ratios of cancer for cohorts prescribed/not prescribed immune-suppressive therapies.
IST: immune-suppressive therapy; Rx: prescription; Yr: year
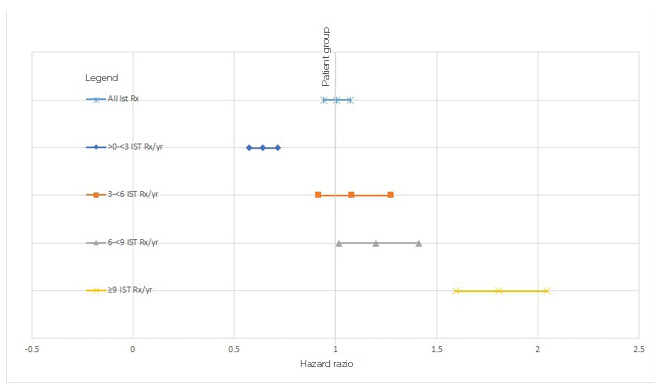
Figure 2: Hazard ratios of cancer for cohorts with restricted comorbidities, prescribed/not-prescribed immune-suppressive therapies.
A descriptive analysis was also taken of the locations of cancer for the non-IST cohort and the IST cohort. The most common cancer within the IST group was skin cancer, followed by breast, prostate, lung, then lymphoma. Within the non-IST group, colon cancer replaced lymphoma as the fifth most common cancer.
DISCUSSION
The results of this large historical cohort study indicated that prescription of one or more of methotrexate, ciclosporin, azathioprine, or mycophenolate in patients with moderate or severe AD in primary care was not associated with a greater risk of being coded for cancer, as compared with no IST prescription. However, there was evidence that higher prescription numbers per year was associated with an increased risk of cancer, both with prescriptions within defined categories, and on a prescription/year basis. Caution must be applied to this interpretation, as patients who are prescribed ISTs will have increased vigilance from both primary care and secondary care, with frequent blood tests compared to patients on topical therapies, and will be more likely to be diagnosed through monitoring of white cell count, liver function, and other markers for cancer. In addition, within the main analysis cohort, treatment decisions around other comorbidities requiring ISTs may not take into account a patient having AD.
Further study is required to explore the association of cancer risk to IST use. Lymphoma37 and skin cancer have been associated with methotrexate38 and azathioprine use, and examination of particular types of malignancy may show stronger signals. The findings by Mansfield et al.12 identified lymphoma as a particular pathology associated with IST use. The authors noted that a descriptive ranked analysis showed that lymphoma displaced colon cancer as the fifth most common cancer code, but requires further analysis to determine significance. Additionally, differentiating between the different types of ISTs will provide further guidance to which may confer a higher malignancy risk. The authors also noted that in the baseline characterisation of patients, the prevalence of psoriasis was 6%. Since psoriasis and AD are considered as opposite poles of T helper activity, it would also be worth exploring the diagnostic pathway of these patients.
The authors also noted that the mean age at IST prescription may be considered to be higher than expected, from a disease that is traditionally considered to be a disease of childhood with some persistence until adulthood.39 However, it has been reported that one in four adults report adult-onset disease, and AD typically follows a relapsing pattern, which can emerge in later adulthood.40,41 The proportion of adults with moderate or severe AD is consistent with figures of 1–3% of total patients within CPRD. Another possibility for this higher age could be the influence of conditions such as rheumatoid arthritis or inflammatory bowel disease, commonly treated with ISTs. However, in the non-IST cohort, the median age of patients with AD was 44 years, at the time of potent, very potent, oral corticosteroid or tacrolimus prescription. The additional analysis examining patients without comorbidities still showed a median age of 43–44 years, indicating that AD presentation in adulthood was not uncommon.
STRENGTHS AND WEAKNESSES
The study has several important strengths relative to prior work. This study utilises a large primary care database covering 20% of the UK population, with a 5-year follow-up period exceeding the follow-up period of prior randomised control trials. Previous work examining malignancy considered patients without consideration of IST prescription.
The limitation of this study is inherent to its nature as an historical study. Despite extensive quality control and validation, records collected in CPRD were collected for routine clinical purposes. As such, some degree of inaccuracy and incompleteness may be present, and the inability to control for potential confounders and variables not recorded in the database.
The authors noted that secondary care prescribing is not captured in CPRD. Patients are initiated on IST by their dermatologists prior to primary care prescribing until the dose is stabilised, typically within 2–8 weeks of initiation, meaning it is likely that the index date for IST prescription is delayed, and time to cancer events underestimated. Other treatments in secondary care, such as ultraviolet light therapy, may also increase the risk of cancer, and are not well documented within CPRD.
The focus of the study was on patients who have relevant dermatitis codes at or after 15 years of age. Many patients with moderate or severe AD will present and may resolve prior to this age, and were not included in this study.
Amongst the practices where CPRD collect data, there are those who do not participate in shared care; thus, the authors did not capture any of their IST prescriptions, and they appeared within this study as part of the non-IST cohort.
As data on medication usage were not captured in CPRD, the current study was unable to control completely for adherence to prescribed ISTs.
Prescriptions for ISTs may not have been specifically for AD. The authors controlled for this by matching on comorbidities where ISTs are prescribed in primary care, and through the secondary analysis where these conditions were excluded.
ETHICS
This study complied with all local and international laws and regulations, including ICH E6 Guidelines for Good Clinical Practices, and the study protocol was approved by the Independent Scientific Advisory (ISAC) committee.






