A highly-effective pharmacovigilance function is a rising value differentiator for pharmaceutical companies, but what challenges are being faced, and what can be done to help pharma’s first responders keep patients safe?
Words by Jade Williams
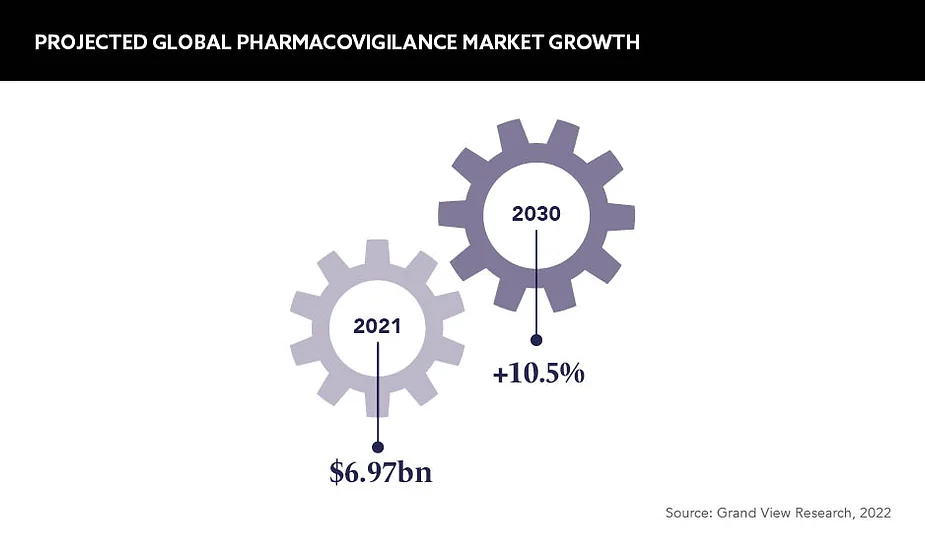
Historically, the value of pharmacovigilance has been greatly overlooked. A successful pharmaceutical company is identified and ranked against the productivity of other functions, but as the valuation of pharmacovigilance is now on the rise – estimates suggest the global market will grow from 7.8bn USD in 2022 to 17.36bn USD by 2030 – this is gradually changing.
“The safety of products is now much more of a differentiator,” says independent pharmacovigilance consultant James Whitehead. “During the pandemic, we saw people stop taking one vaccine to take another because they had heard about a potential adverse event related to the product.”
While drug safety has always been a priority for the public, it dominated conversations about COVID-19 vaccinations when they were first approved and served as a key driver for vaccine hesitancy or preferentialism. This feeds into the key growth driver behind the rise in value of pharmacovigilance: an increasing incidence of adverse drug reactions (ADRs) being reported.
The importance of reporting
Knowing that end users are not only more aware of the possibility of reporting reactions from drugs, but also aware of open-access databases listing ADRs from brands they use, companies should seize this opportunity to invest in their pharmacovigilance teams, mitigating harm to the public while protecting their brand image, too.
The safety of products is now much more of a differentiator
Although awareness of how to report ADRs to the industry is increasing, Dr Ian Gray, Director of Regulatory Affairs and Quality Assurance, UK and Ireland, Ipsen, believes that people still need an extra push in the right direction. “The challenge is people reporting,” he says. “At the moment, it is voluntary for HCPs and patients to report, but mandatory for manufacturers.” He highlights education initiatives and awareness campaigns as powerful tools to help people understand how to report and why it is so important to do so.
The issue of low-quality data
While a logged ADR is fantastic first step, this does not necessarily mean that the issue can be immediately solved. A key challenge hampering the detection of trends lies in a reliance on free text fields in reporting software, leaving users to their own devices on what they wish to write.
For example, answers submitted by the public can often be vague, inputted simply as ‘headache’ with no elaboration, leaving large gaps in the collation of data on a drug’s report. Answers could also detail a variety of reactions or ailments, clouding the data as it is unclear what is actually a result of the medicine taken. “You have all this data, but how do you know what is associated with your medicine?” asks Whitehead. “It’s as much an art as it is a science when having to apply statistical analysis to identify an event.”
On the other hand, the reporting of adverse events during clinical trials functions effectively in most cases. Amro Turki, Pharmacovigilance and Medical Information Manager, Consilient Health, explains: “Within a clinical trial you have a doctor that is actually reporting the information on ADRs to you, and they have to provide you with a certain set of information that you can then follow up on to gather more data.” While the industry cannot expect the general public to report issues in as much detail as an HCP, encouraging a detail-lead mindset when submitting forms could lead to better results in data tracking.
The field needs to evolve from pharmacovigilance to patient safety to treat patients holistically and ensure their overall safety
One such method of obtaining necessary detail is through the adoption of dedicated drug safety platforms such as ‘Reportum’ from the pharmacovigilance company MyMeds&Me. This enables patients to report symptoms by clicking the area of the body that has been affected, which then prompts them to provide additional specific details at each step. Simplifying the reporting process by introducing platforms such as this could greatly improve the quality of data received from the public, improving specialists’ abilities to glean more accurate readings from the results.
In addition, data reporting can be muddied by duplicates when information is collected from multiple sources – when a therapy holds marketing authorisation in both the US and UK, for example. “If departments are not in sync, the US department will report the same event as the UK department, which negatively affects the global database,” states Turki. Improving existing algorithms to negate the duplication of reports would therefore greatly increase the accuracy of pharmacovigilance reporting, helping medicines to stay as safe as possible.
Evolve to stay relevant
Speaking about the UK market specifically, Gray states that pharma must continue to partner with the MHRA to iron out existing issues and keep improving ADR reporting in the long term. “This collaboration is working well at the moment, but we must maintain that hand-in-glove relationship where we work with industry groups to ensure we’re moving at the same pace. We must make sure the foot doesn’t come off the gas.” The same could be true for collaborations with other regulatory organisations around the world, too.
There is certainly an evolution to be seen within the field, so much so that Whitehead warns: “Pharmacovigilance is, in itself, fading. Very few products come as just a medicine anymore – they come with an application or a device. The field needs to evolve from pharmacovigilance to patient safety to treat patients holistically and ensure their overall safety.”
Keeping up with the pace of innovation will be crucial to ensuring the value of the pharmacovigilance function continues to rise, and it is hoped that with more attention and investment into pharmacovigilance teams across the world, they will be able to become even more effective at keeping the public safe.