Decentralised clinical trials have a clear place in the pharma industry’s future, but as the pandemic gradually drops back, there are hurdles stopping them from reaching the finish line of wider adoption. How can emergency measures be translated into permanent solutions to increase the power and number of these trials?
Words by Isabel O’Brien
During the pandemic, the function of people’s homes morphed and multiplied. The public sphere became a feared and unoccupied space, and stay-at-home orders were issued to control the virus and protect the most vulnerable in society – something that won’t be forgotten in a hurry.
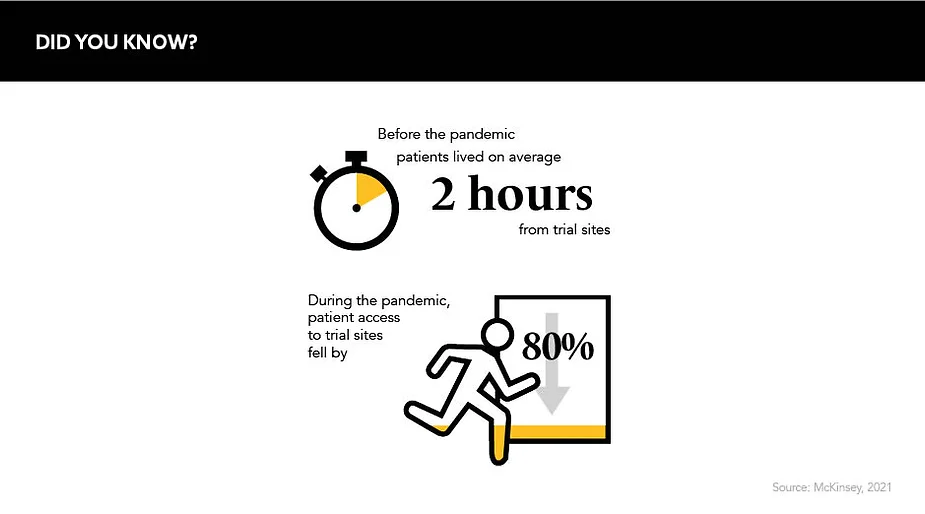
To lessen the burden of this new way of living, services sprung up to enable the direct delivery of everything from produce and pastimes to health advice. But, perhaps, nothing was more innovative than the efforts made to relocate clinical trials into participants’ homes – a necessary measure after patient access to trial sites fell by a staggering 80% in early 2020.
Slow and steady didn’t win the race
Before COVID-19 hit, many pharmaceutical companies had been weighing up the pros and cons of lifting clinical trials from the lab to home locations – patient burden and a general lack of population diversity acting as catalysts – but many were making incremental changes that did not tackle historical recruitment issues.
“Prior to the pandemic, most biopharmaceutical companies were thinking about how to ‘modernise’ clinical trial conduct through technology and other established non-traditional approaches,” confirms Marie Rosenfeld, Global Head of Clinical Sciences, Astellas.
Updates included electronic consent forms, home health nurses and ‘bring your own device’ schemes for electronic clinical outcome assessment and diary data collection, according to Rosenfeld. All great steps in the right direction, but far from adding up to fully decentralised solutions.
Then, the pandemic sparked a seismic change in how the industry conducted clinical research. “COVID-19 pushed companies and regulators to rethink traditional clinical trial norms,” she explains. “With no other option, things that had been deemed as complex to even impossible, became absolutely necessary.”
Until there’s a better harmonisation achieved, this can influence the choice of countries to include in a particular trial
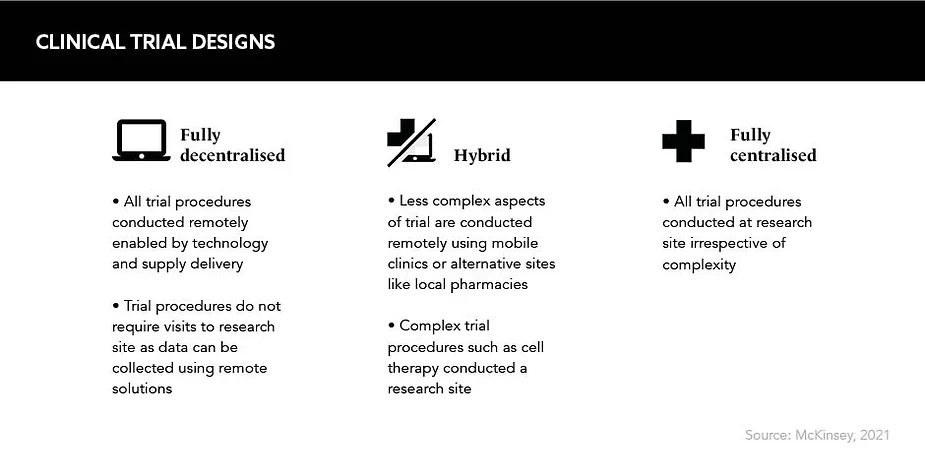
Innovative technologies and platforms that had been waiting in the wings – such as remote consent and patient monitoring, video conference assessments and even remote mobile blood drawing services – were rapidly harnessed by pharma to facilitate decentralised clinical trials (DCTs) in a bid to prevent disruption to medical research.
“The COVID-19 pandemic was a watershed moment for the life sciences industry and specifically for the methodology of clinical trials,” says Van Zyl Engelbrecht, Director of Clinical Operations, Lindus Health. But as DCTs were launched as an emergency measure, there are now historical barriers to be addressed to ensure remote trials can continue into the future.
Country inconsistencies
To unlock the power of remote trials and reap the highest rewards they offer, companies must launch decentralised, multi-country studies that enable a diverse participant population to take part from all over the world. However, many countries – even those within the EU – have differing regulations that are blocking this possibility.
“The pandemic resulted in countries putting exceptions into place,” says Eric Klaver, Decentralised Clinical Trials Regulatory Director, IQVIA. “Those exceptions are not yet embedded into the legislation, and with the pandemic changing course, some of those exceptions are discontinued.” For example, German regulation dictates that a citizen cannot take place in a remote trial based in another country when the drug is delivered directly to their home, but they could take part if they travelled to the site in that country to collect the medication themselves.
There are also contradictions on the use of electronic media and processes to obtain informed consent from trial participants, otherwise known as e-consent. The FDA issued guidance on e-consent in 2015, the MHRA followed suit three years later and the EMA only shared its view in 2021. “Until there’s a better harmonisation achieved, this can influence the choice of countries to include in a particular trial,” says Klaver. As a result, most fully remote trials are currently based in the UK and the US exclusively.
Still, the regulatory outlook is promising, as Engelbrecht says: “Fortunately, all three major regulatory authorities are addressing the components required to conduct DCTs, and the direction of travel is for more hybrid and DCTs in life sciences research.”
Other hurdles include ensuring data privacy and protection on virtual trial channels, elevated dropout rates and cultural differences between countries. To this end, certain trials will be more suited to decentralisation than others. In terms of a drug-specific context, a well-characterised drug with few adverse events in a mild indication would be a safe place to start.
Go forwards, not back
Replicating a lab inside a patient’s home is challenging, therefore it is crucial for differing regulations and other hurdles to be cleared so innovation around safety and accuracy can flourish. “We cannot go back; we need to move forward,” declares Klaver. “Things that have proven possible and successful should get incorporated into legislation. This just cannot wait.”
While fully decentralised clinical trials are needed now, expert estimations for wider adoption are conservative. For both Engelbrecht and Rosenfeld, wider use is still a few years away. “Decentralised trials will become commonplace for certain types of clinical trials in the next three to five years,” says Rosenfeld. But, as with any hurdle, the first step is seeing it, the second is clearing it and the third is the home straight to victory. The industry is on track but must not take its eye off the finish line.