The collection of real-world data for rare diseases can prove a troublesome task for medical affairs and researchers contending with smaller patient populations dispersed around the world. How can pharma harness the power of real-world data strategies to improve the understanding of, and support for, rare diseases?
Words by Jade Williams
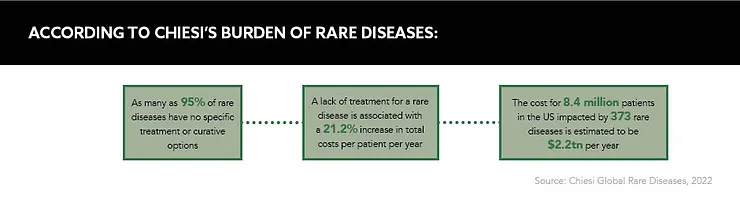
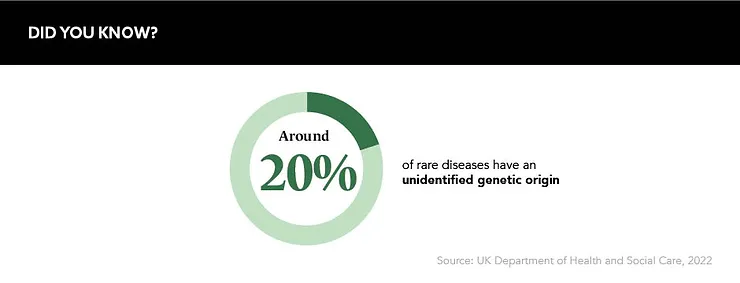
Rare diseases: the clue is in the name. Affecting fewer than five in 10,000 people and spread across the planet, these small patient populations with highly unique needs can spell difficulties for drug developers working with limited data sources.
“Literature says there are around 8,000 rare diseases out there, but nobody really knows the real figure.” comments Iris Himmelhan, Digital Strategy Lead, Rare Early Diagnosis, Takeda, at Reuters Pharma 2022. “There’s not a lot of real-world data (RWD) out there for rare diseases – rare, by definition, is rare.”
With such an extensive range of specifications across a span of potential therapeutic areas and countries, the information available to researchers can indeed prove scarce, and clinical trials arduous to conduct. What’s more, patients can often be under- or misdiagnosed by healthcare professionals, leading to further data capture issues for different conditions and treatment efficacies outside of trial settings.
Availability and appropriateness
While technology is progressing in leaps and bounds to collect ample RWD from wearable devices and the like, this data is not easily implemented into pharmaceutical processes. The data gathered can be unstructured, highly variable and increasingly difficult to control. Additionally, Elizabeth Kinter, Head of Global Access Strategy, Takeda, also speaking at the Reuters event, notes that “in the end, there’s probably not a lot of sources for being able to collect this data” and ensure it is useable. “If it is available, a key question is ‘can a manufacturer actually gain access to this?’,” she adds.
“It’s a bit of a chicken and egg thing,” Himmelhan elaborates. RWD is needed to develop new and important treatments for people with rare diseases, but efficient, easily translatable data can only be produced once these patient populations are found and entered into the pharmaceutical pipeline. Put simply, someone must make the first move.
There’s not a lot of real-world data out there for rare diseases – rare, by definition, is rare
One form of tried and tested RWD collection, however, comes in the form of adverse event reporting. “If we’re looking at efficacy [of collection], any sort of safety event that happens would have to be reported anyway,” Kinter notes. Leveraging this readily available data could form an important basis for RWD collection, giving medical affairs professionals a solid base for analysis and then researchers a starting point to improve rare disease treatments for those affected.
Once a source of data has been identified and deemed appropriate for clinical use, “it’s very important to look at the datapoints longitudinally,” continues Himmelhan. “Data that’s not longitudinally connected is pretty much useless because you need to understand the natural history of the disease. Without longitudinal data, this is pretty much impossible to move forward with.”
A regulatory revolution
Besides actually gathering data from rare disease patients, Himmelhan notes another concern in the form of regulatory hurdles. “I refuse to accept the way things are right now,” she comments. “The way RWD is commercialised – for example in the US where the big players own and sell the data – do we really want that to be the same here in Europe?” A huge underlying factor in gathering RWD from patients is a company’s ability to win their trust, and that’s something the pharma industry has been struggling with for decades thanks to media portrayals and various high-profile scandals.
Data is arguably the most valuable commodity in the modern world, or it will soon grow to be. Whether given, bought or sold, RWD is worth its weight in gold to consumer data brokers. Himmelhan argues this should not be allowed to happen within pharma, and that governments need to act fast to prevent Europe’s landscape shifting toward a capitalistic playground. The industry should come together to “make governments start thinking about putting guardrails in place, before it becomes the wild west”, she says.
Make governments start thinking about putting guardrails in place, before it becomes the wild west
To abate regulatory concerns that could be expressed by patients, Kinter recommends keeping early, behind-the-scenes discussions broad and collaborative, conducted “in partnership with medical teams and legal, which always have a seat at the table”. Having transparent processes when approaching RWD collection will enable researchers to develop a firm foundation of trust with patients, allowing for the greater leveraging of more datapoints.
A return to collaboration
“Rare diseases are often chronic and progressive,” states Himmelhan. “What we provide is not always medication, but also treatments or medical care that could potentially improve quality of life.” It is important that all pharma stakeholders always remember the crux of their duty to patients, and work to improve RWD strategies for the greater good of those suffering from rare diseases.
During the pandemic, Himmelhan argues the industry “shared a common purpose, but somehow it feels that right now we’ve stepped back”. The collective effort to improve conditions for patients seems to have fallen off the agenda for many companies as they return their gazes inwards, with less shared data and collaboration. To truly utilise and embrace RWD to spearhead new research and innovative care for people with rare diseases, developing a network of shared resources to work on in collaboration – covering all angles and ensuring the best for patients – will surely be of great benefit.