Addressing global access to medicine, particularly in LMICs, poses a significant challenge. As COVID-19 vaccines are distributed, how can the pharmaceutical industry ensure they reach vulnerable populations in these countries while protecting patent rights?
Words by Michaila Byrne
Access to essential medicines in low-to-middle income countries (LMICs) is one of the most complex, yet critical, ongoing challenges facing the pharmaceutical industry today; one that has only been amplified following the development of COVID-19 vaccines.
As we roll-out these lifesaving products, it is of the utmost urgency that we reflect on what is being done to combat accusations of ‘vaccinationalism’ and apply actionable insights, guaranteeing access for our most vulnerable populations. The old adage ‘a chain is only as strong as its weakest link’ is more pertinent than ever, a proverb the industry must keep top-of-mind as they begin distributing vaccines across the globe.
Vaccine companies should negotiate with COVAX, quickly move vaccines into the queue for WHO approval, and ramp up supply
Barriers to distribution in LMICs
Currently, much of the coverage and reality of vaccine distribution suggests that it is only reserved for a select few who can afford them. “The sad truth of vaccine access is that it takes time, sometimes decades, between introduction and significant uptake in high income countries before there is widespread availability and implementation in LMICs,” outlines Jerome Kim, Director General, International Vaccine Institute.
“In short, there are coordination issues — prior contracts, manufacturing, approvals, distribution — that are impairing the current roll-out internationally.” The ability to swiftly distribute any medicine is greatly dependent on respective local health policies, financing, resources, as well as pricing processes in individual countries. When it comes to vaccine access in particular, decisive action must be taken promptly for the sake of public health.
As Ulf Staginnus, Senior Vice President, Head of Global Market Access and Pricing, Ipsen, warns: “Shortcomings of access to vaccines in a pandemic situation such as today obviously implies an increased risk of further global spread of infections and a prolongation or reimportation of the problem.”
Strategies for improved access to medicine
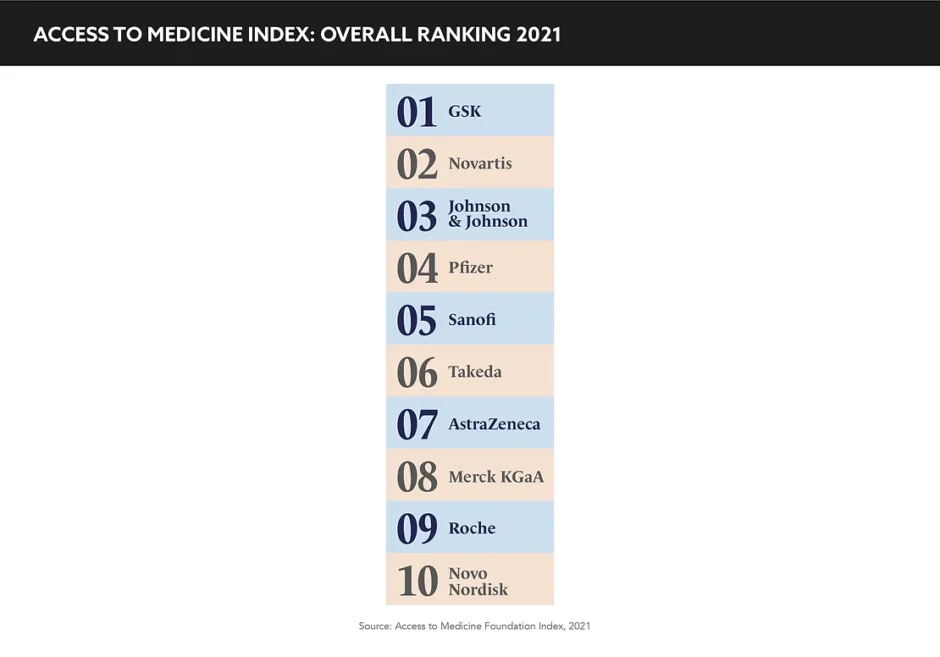
Access to medicine has been an ongoing discussion even before the outbreak of the pandemic, the breadth of which is detailed in ‘The 2021 Access to Medicine Index’, which highlights concrete, tangible opportunities for pharma companies to make products more accessible in LMICs.
Claudia Martínez, Research Programme Manager, Access to Medicine Index, advises: “Companies can adopt a number of different strategies when it comes to delivering and improving access and creating equitable pricing strategies. They can also manage their intellectual property responsibly and wave patent rights, engage in voluntary licensing, and also engage in donation programmes.”
For example, companies like Takeda and GSK encourage their senior leadership teams by including access-related incentives in annual bonus plans, a model that ensures access is systemically applied. The report did conclude that there is evidence of pharma integrating access into business practices, but most of these initiatives are focussed on only specific products and apply to only certain countries.
They can manage their intellectual property responsibly and wave patent rights, engage in voluntary licensing, and engage in donation programmes
Global collaboration
From a manufacturing perspective, capacity and production must be higher on the agenda, coupled with flexible payment plans, contracting, and supply policies.
According to Staginnus, in many ways, the approach to vaccine roll-out for the most vulnerable and disadvantaged populations should not be dissimilar to any other medicine: “The main task is to ensure capacities and manufacturing collaborations are maximised to be able to supply sufficient doses worldwide as fast as possible… it is a matter of great commitment of all parties involved, dedication, and for once a focus on outcomes, not just the price for a vaccine.”
Fortunately, with the launch of COVAX, there is a path to guaranteeing fair and equitable access for all countries, but it is vital that the industry rally and sincerely commit, with Kim echoing: “Be committed to COVAX… vaccine companies should negotiate with COVAX, quickly move vaccines into the queue for WHO approval, and ramp up supply through contract manufacturing and licensing arrangements.”
Pharma’s approach must advance and scale to ensure that it is not only the upper middle-income countries that are benefiting from access strategies, but that we are prioritising the poorest as well.
Embedding access into strategy
If any point of a chain is weak, the strength of the whole chain suffers and fails to perform its function; without equitable access, there are no winners, and the chain risks collapse altogether. If pharma can systemically imbed access into their business strategies, they can transform this moment into a global health triumph.
To achieve this level of unity and solidarity, access to medicine must be understood and treated not as an afterthought, but as something that is it at the core of what companies do and underlines their purpose.