The challenges faced by the transgender community are vast, but the pharmaceutical industry has the power to drive better health equity for these patients
Words by Isabel O’Brien
The pharmaceutical industry prides itself on being patient centric, but when it comes to transgender-inclusive healthcare, its approach is largely failing to hit the bullseye. From internal awareness to clinical trials to medical education and more, the pharma industry can play a role in helping this underserved community to be better supported on their healthcare journeys.
As Jennifer Petter, Founder and Chief Innovation Officer, Arrakis Therapeutics, explains: “While there are complexities, many categories of patients offer complexities.” Petter transitioned in 2018, and she has a message for the pharma industry when it comes to allying with the trans community: “Just do it.”
Taking the initiative
“Pharma is where pretty much the rest of the world is – we don’t know where to start,” admits Darshan Kulkarni, Life Science Entrepreneur and Regulatory and Compliance Attorney for the Life Sciences, The Kulkarni Law Firm.
While there have been civil rights victories for the trans community in recent years, including a landmark US ruling in 2020 that made it illegal to discriminate against people on the basis of sexual orientation or transgender status, the issues they face can be under-recognised and under-supported by those outside the community.
The global trans population is “battling multiple wars at the same time”, clarifies Kulkarni. Therefore it is key for the pharma industry to identify and tackle associated health inequities directly, rather than waiting for the community to lobby it into action.
First, look inwards
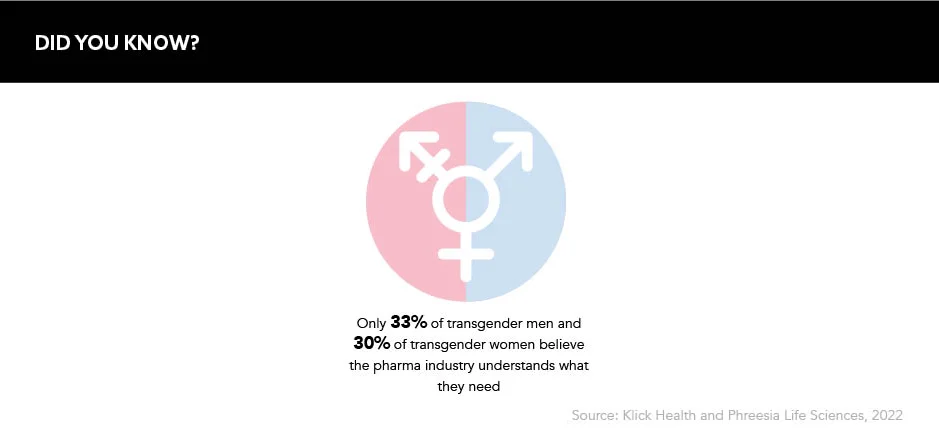
Pharma companies seeking to be an ally to the trans community should take a multifaceted approach, and this starts by reflecting inwards at how they treat trans issues within their organisations. “Companies should start by being honest about what they don’t know when it comes to trans issues,” says Jane Brearley, CEO and Founder, Intent Health, and a member of the Healthcare Businesswomen Association (HBA) Europe’s U-FACE Affinity Group. “If you want to improve awareness, you need to know what the issues that need attention are.”
Examples include educating employees on the correct use of pronouns and setting up dedicated employee resource groups (ERGs) for LGBTQ+ staff. A recent report by the Biotechnology Innovation Organization found that in 2021 only 48% of biotech organisations had set up ERGs, highlighting the need for others to step up and do the same. The study also identified that many did not have a dedicated ERG for LGBTQ+ issues, with only 25% of smaller biotech organisations having one in place.
While there are complexities, many categories of patients offer complexities – just do it
Clearly, there is work to be done, and Kulkarni believes pharma leaders must trailblaze the change from the top down. “It needs to be communicated from the C-suite that this is an important community that we want to address their needs for,” he says. And Jane Finch, Founder and Diversity and Inclusion Specialist, Time for Inclusion, and HBA member, adds, “only after educating yourself, consult trans colleagues and ask them how they wish to be treated in the healthcare system”, asserting that the first step for leaders must always be getting their own and their organisations’ attitudes up to scratch.
Diversifying clinical trials
As it stands, the mechanisms for enrolling trans people in clinical trials are weak. This not only means that trans patients are not represented in historical clinical evidence for already approved therapies, but it also means they’re unlikely be counted in new trials too.
“To be fair, the New England Journal of Medicine estimates that around 0.7% of the US population is trans, so only the largest trials are likely to include statistically meaningful numbers of trans patients,” Petter reasons. However, many of these larger trials are not enrolling trans patients at all, although there are notable exceptions to this rule, such as Moderna’s COVID-19 vaccine trial in 2020.
To become more inclusive of the trans community, it is crucial for clinical trial organisers to review their approach to enrolment of minority group. “The pharma industry needs to ensure trial environments and teams are welcoming and inclusive, so trans people actually feel comfortable enrolling in the first place,” says Brearley. This could be achieved by simply ensuring the language used on enrolment forms accommodates for non-binary identification.
If you want to improve awareness, you first need to know what exactly the issues that need attention are
“By not explicitly including trans patients in your care spectrum, inclusive and exclusive criteria and research, you are making it more difficult for physicians and other prescribers to safely and efficaciously use a certain drug for a specific patient.” Kulkarni emphasises. He believes the FDA and EMA should also be making more “overt requirements” for greater diversity in clinical trials to usher in change.
There is also very little research by pharma into how hormone replacement therapy (HRT) could be impacting patients with other conditions, as HRT for this usage is prescribed off-label. While some instances of research into the effects of HRT have been noted – research published in the Journal of Clinical Endocrinology and Metabolism states, for example, that there was no difference in the rate of type 2 diabetes in cisgender people and trans people undergoing HRT in 2022 – pharma companies could help in this realm by sponsoring similar types of studies.
Medical education and marketing
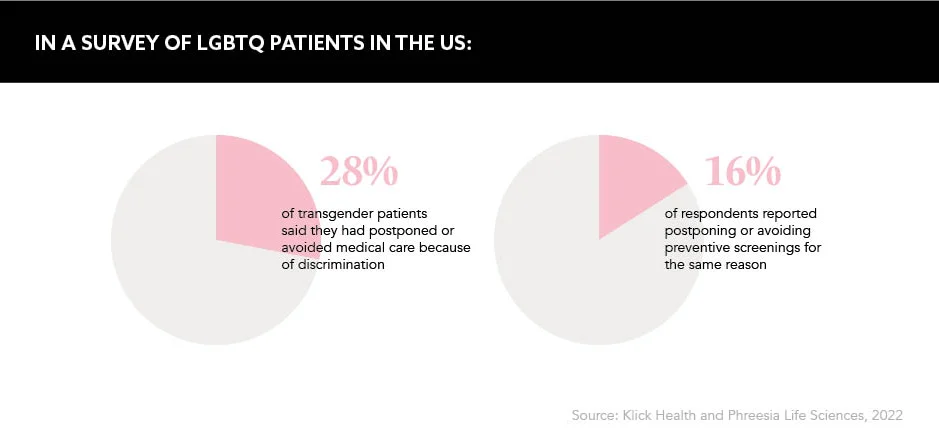
Despite the trans community becoming more visible in popular culture in recent years, stigma is still prevailing in healthcare delivery. In the UK, 70% of trans people have reported experiencing transphobia from their primary care provider, with 14% refused GP care because they were trans.
Consequently, medical affairs professionals have a duty to challenge bias with targeted education drives. “Build relationships with HCPs and clinics that have a high percentage of trans patients,” urges Petter. She also states that it is important for MA include information on drug-drug interactions with common drugs used by trans patients such as spironolactone – which suppresses male hormones – or oestrogen.
Pharma is where pretty much the rest of the world is – we don’t know where to start
In addition, marketing teams can help break down stigma by working with trans individuals on product and disease awareness campaigns. Pharma can look to Virgin Atlantic’s latest TV advert for inspiration. The company gives the cabin crew pronoun badges and lets them wear the uniform that matches their gender identity. “There is a scientific impact, then there is the societal impact, and marketing and PR really helps with that part of it,” asserts Kulkarni. A survey conducted by the UK’s Channel 4 in 2020 indicated that just 0.3% of adverts in the UK feature trans people, revealing a notable gap to be addressed.
The bottom line
There is huge scope for pharma to transform itself and healthcare systems into more trans-inclusive spaces, and this must be approached via the industry’s commitment to patient centricity, as well as its internal business strategies. As Petter reflects on her own experience, she asserts how life sciences is certainly up to the task. Describing pharma and biotech as “a highly educated community that values data and fairness and enlightenment”, she continues to say that transitioning was “a very positive experience” for her personally.
To improve health equity for trans individuals, the pharma industry must tackle inequities across its own organisations, particularly its research funnels. Only then will it be able to impact how healthcare systems serve this community, dismantle stigma and improve health outcomes for trans patients overall.