Despite moves in a positive direction, opioid abuse shows little sign of abating, but there’s a huge opportunity for the pharmaceutical industry to play a role in addressing the crisis
Words by Saša Jankovic
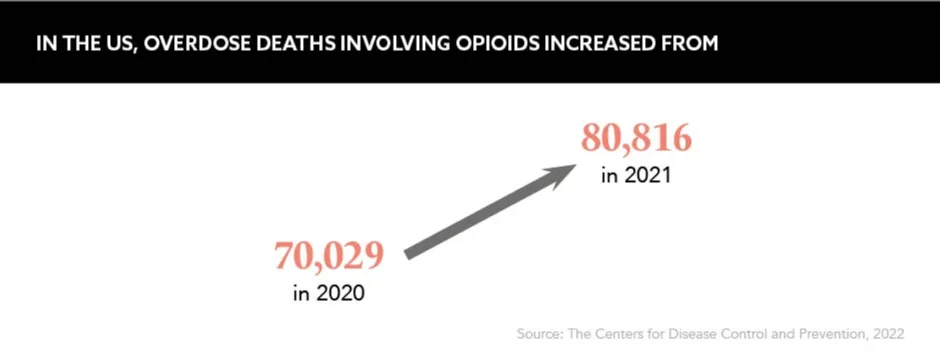
The past 25 years have seen an increase in opioid morbidity and mortality in the US and Canada with 2020 the worst year on record for fatal opioid overdoses. Figures for the US show opioid toxicity deaths climbed 37% to 70,168 in 2020 – up from 51,133 in 2019 – with the Centers for Disease Control and Prevention estimating that 75,387 deaths occurred during the year ending 30 May 2021. In Canada, the incidence of fatal opioid overdoses increased by 72% from 3,668 in 2019 to 6,306 in 2020, with a further 3,515 deaths reported in the first half of 2021.
While the UK does not have the same levels of opioid use or abuse as North America – or a comparable population size – 2020 still saw 2,263 opiate-related deaths in England and Wales. The issues are undoubtedly widespread.
Sector reparation
The ongoing opioid crisis in the US has been linked to many well-publicised factors but, as well as being party to these, the pharmaceutical industry has taken steps towards reparation. For example, settlements have been paid out by companies such as Purdue and Teva Pharmaceuticals in recent months, with the aim of increasing access to life-saving treatments for people suffering from opioid addiction.
“At Harm Reduction Therapeutics (HRT), we are driven by a commitment to help prevent opioid overdose deaths by working to make naloxone available to everyone,” said Michael Hufford, Co-Founder and CEO, HRT – the non-profit pharma company benefitting from Purdue Pharmaceuticals’ $11m financial settlement in March 2022. “With lives lost every day from opioid overdoses, ensuring broader access to affordable, over-the-counter naloxone is an urgent priority. We are continuing to advance the development of OTC naloxone nasal spray to help communities across the [US] affected by the opioid crisis.”
Ensuring broader access to affordable, over-the-counter naloxone is an urgent priority
While this progress and development is undoubtedly welcome, repairing reputations can take a considerable amount of time, and with this particular drug type there are some additional hurdles to overcome. A notable comparison is with the tobacco industry. “There is a welter of independent evidence that proves e-cigarettes and similar products are at least 90% safer than cigarettes,” comments Harry Shapiro, Director, DrugWise, “but because the tobacco industry has a stake in these products, much of the international public health community led by the World Health Organization refuse to accept the evidence [and] attempts by the industry to support stop smoking efforts have been attacked as the industry trying to make up for past crimes. So, you see the problem that pharma faces in trying to rebuild trust regarding opioids.”
Dr Andre Waismann, Head of the Accelerated Neuroregulation (ANR) Unit, Barzilai Medical Center, Israel, and Director, ANR Clinic, Florida, US, believes the pharma industry became a scapegoat for what he calls “the failure” of academic institutions to develop effective treatments for opioid dependency. This is “a reversible neurological medical condition”, he says. “When this issue became an epidemic and damaged the lives of people, the scapegoat became the pharmaceutical industry producing the substances. This is akin to blaming the car industry for road accidents, which would be absurd.”
The solution for the pharma industry, according to Dr Waismann, is also its biggest challenge, and that’s education. “I believe all the legal lawsuits against big pharma were not well conducted because most of the experts expressing their opinions on addiction and dependency were just ignorant with their statements,” he says. “I believe that once all this ignorance is put aside and people recognise opioid dependency as a reversible medical condition and not a chronic relapsing illness the pharmaceutical industry will be completely off the hook.” Only time will tell whether this optimistic viewpoint holds true.
Future steps
In the meantime, although making good financially is in no way the end of the story for the people and societies most affected by opioid abuse, this action does give pharma – and others – a solid starting point for positive change. There are countless possibilities such as committing to influencing regulatory reform, prescribing practices and opioid stewardship.
For example, The Stanford–Lancet Commission on the North American opioid crisis, which brings together diverse Stanford scholars and other leading experts to propose solutions to the opioid crisis domestically and attempt to stop its spread internationally, claims that because the epidemic of opioid addiction and overdose “emerged from, and is still to some extent being fuelled by, legally prescribed opioids, policy responses need to be uniquely tailored to that reality”.
In its recent report, the Commission warns over a million more people will die of opioid overdoses in the US and Canada by 2029 unless direct-to-consumer advertising by drug companies is banned, alongside an end to pharma’s funding of continuing medical education programmes.
The scapegoat became the pharmaceutical industry
Despite critics saying the FDA’s 2016 review of prescription painkillers had lost sight of the recommended reforms therein – such as potentially removing some drugs from the market – for its part, the FDA says it has “made combatting opioid misuse, abuse and addiction a priority” over the last few decades.
However, admitting that “we can do more”, August 2022 saw the FDA release its ‘Overdose Prevention Framework’ to undertake what it calls “impactful, creative actions to prevent drug overdoses and reduce deaths”. This is part of its broader work to address the overdose crisis, in which it is conducting a review of its actions and decisions on opioids “to obtain an analysis of FDA’s key regulatory policies and decisions, including labeling, and recommendations to improve our future approach to support appropriate use of opioid analgesics”.
As the results of the review are awaited, there is huge opportunity for pharma to get ahead of the game and run with some of these actions, too.