Meeting Summary
Therapeutic basal insulin has evolved considerably over the past 90 years. A series of landmark innovations has led to the availability of therapies that closely mimic the physiological effects of endogenous insulin and serve as an invaluable addition to the treatment armamentarium for diabetes. Advances in basal insulin have led to the development of the newer basal insulin analogues, namely insulin degludec and insulin glargine 300 U/mL (Gla-300). The desirable pharmacokinetic (PK) and pharmacodynamic (PD) properties of these basal insulins, such as a prolonged duration of action (≥24 hours), may translate into a number of clinical benefits for the patient e.g., a simple, once-daily injection schedule and flexible injection timings.
The technologies supporting patients with diabetes have also evolved considerably in recent years. Continuous glucose monitoring (CGM) can provide insights into some of the challenges faced by patients with diabetes, e.g., glycaemic excursions and the impact of injection time, and may become an alternative to the current gold standard glycated haemoglobin (HbA1c). Real-world evidence is also providing fresh perspectives on the effectiveness of basal insulins in clinical practice. Today, innovative methods for real-world evidence collection, analysis, and interpretation are helping to generate robust datasets with external validity. Taken together, these innovative approaches are generating an integrated evidence base that is improving our understanding of how basal insulin therapy can be optimised for the benefit of our patients with diabetes.
Symposium Overview
Doctor Elizabeth Seaquist
Diabetes care is evolving. Advances in our understanding of diabetes pathophysiology and treatment now permit individualised therapy based on patient-centred treatment plans that provide the best evidence-based therapies, while minimising personal burden. This personalised approach to treatment requires that therapeutic goals go beyond glycated haemoglobin control to include patient identified outcomes of value such as side effects, cost, and minimal interference with daily living.
Introduction
Professor Robert Ritzel
Landmark innovations in therapeutic basal insulin over the past 90 years, including the development of recombinant human insulin and the introduction of long-acting basal insulin analogues, have led to the availability of therapies that aim to closely mimic the physiological effects of endogenous insulin. Current efforts in diabetes management are looking beyond traditional indicators of glycaemic control and treatment efficacy, to consider the overall patient experience. HbA1c represents the current gold standard for assessment of glycaemic control. However, HbA1c measurement does not take into consideration glucose variability, which may be linked to the pathogenesis of diabetes complications and hyperglycaemia.1 Furthermore, glucose variability may impact diabetes management and, in turn, patient quality of life.2 CGM, a method of measuring glucose variability that can provide real-time data, is now a standard of care in the management of patients with Type 1 diabetes mellitus and is increasingly being used by patients with Type 2 diabetes mellitus. Innovation in basal insulins encompasses not only intrinsic improvements in the products themselves, but also how emerging diabetes-related technologies (e.g., CGM) and evidence from real-world studies can together provide an integrated understanding of the effectiveness of existing basal insulin analogues in clinical practice.
Enhancing Confidence with Newer Basal Insulins
Doctor Jeremy Pettus
Key Points
- Innovations in basal insulin have led to the development of the newer basal insulins, namely insulin degludec and Gla-300.
- Clinical studies demonstrate that insulin degludec and Gla-300 are associated with improved PK and PD profiles, and a reduced rate of hypoglycaemia, compared with insulin glargine 100 U/mL (Gla-100).
- CGM profiling and glycaemic variability analyses are providing real-time insights on glycaemic outcomes with basal insulin use in patients with diabetes.
- Taken together, these data should enhance confidence in the use of the newer basal insulins in clinical practice and support appropriate titration for the benefit of our patients.
Innovations and advances in therapeutic basal insulin have led to the development of the newer basal insulins, insulin degludec and Gla-300. Developments in investigational approaches now permit simultaneous and quantitative study of both the PK and PD properties of insulin preparations using the glucose clamp technique (Figure 1).3
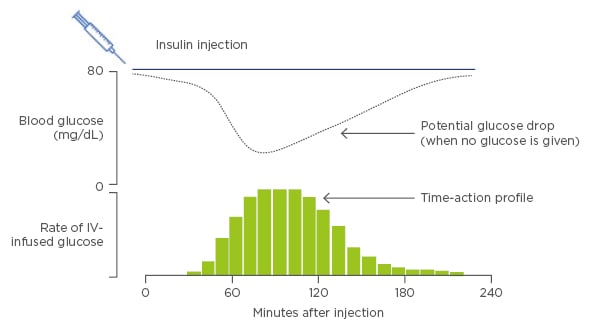
Figure 1: The glucose clamp technique: A means to quantify action of basal insulin.
IV: intravenous.
Adapted from Heinemann and Anderson.3
Preliminary evidence from glucose clamp studies with the newer basal insulins have demonstrated a flatter PK/PD profile, more evenly distributed glucose-lowering effects, and lower between day glucose variability with insulin degludec and Gla-300 versus insulin Gla-100.4-9
Continuous Glucose Monitoring Analyses and the Patient Experience
While PK/PD and clinical studies can provide insights into the effect and efficacy of basal insulin analogues in an investigational setting, in the real world patients with diabetes face many challenges with regard to optimising their therapy, primarily achieving glycaemic control while avoiding hypoglycaemia.10 Although HbA1c represents the current gold standard for assessment of glycaemic trends in different populations, it is not necessarily an appropriate metric for measuring glucose control at the individual patient level, due to the wide range of glucose concentrations and glucose profiles that are available for a given HbA1c value.10 As an estimate of mean, HbA1c based on a measurement cannot provide an accurate report of within-day or day-to-day fluctuations in glucose control and may potentially ‘mask’ episodes of significant dysglycaemia, e.g., hypoglycaemia, which can impact clinical outcomes and patient quality of life. CGM offers an alternative approach for assessing glycaemic control that measures changes in mean glucose concentrations in real time. As daily changes in glucose concentrations vary substantially from one individual to the next, it is important to note that multiple patients with the same HbA1c may exhibit very different CGM profiles.11 CGM profiling was recently published in a randomised, 16-week, exploratory study to compare glucose control in adults with Type 1 diabetes mellitus receiving either Gla-300 or Gla-100, self-administered in the morning or evening.12 Analysis of the obtained CGM profiles revealed that mean 24-hour glucose profiles were smoother with Gla-300 versus Gla-100, irrespective of the injection time (morning or evening; Figure 2),12 findings which were consistent with the known PK/PD profile of Gla-300.6,7
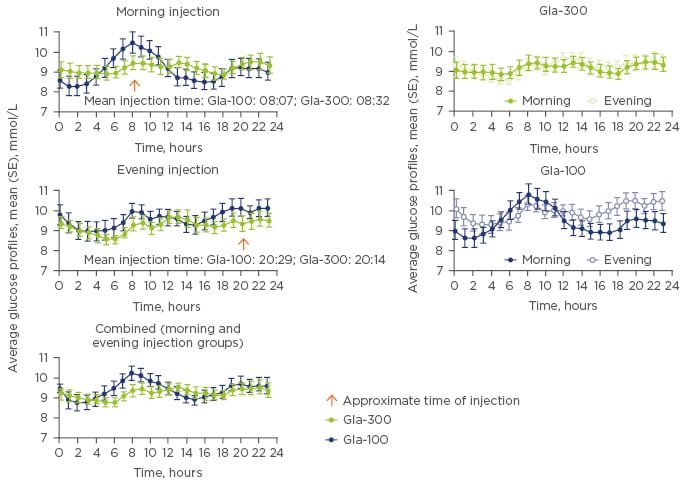
Figure 2: The glucose clamp technique: A means to quantify action of basal insulin.
Gla-100: insulin glargine 100 U/mL; Gla-300: insulin glargine 300 U/mL; SE: standard error.
Adapted from Bergenstal et al.12
Additional analyses demonstrated that Gla-300 was associated with a lower rate of nocturnal, confirmed (<54 mg/dL by self-monitored plasma glucose), or severe hypoglycaemia compared with Gla-100 (four versus nine events per participant- year; rate ratio: 0.45; 95% confidence interval [CI]: 0.24–0.82).12 In terms of glycaemic variability, all metrics for intra-subject, within, and between-day glucose variability were numerically lower for participants receiving Gla-300 versus Gla-100.
Data from CGM profiling and glycaemic variability studies are providing new and important insights on clinical outcomes with basal insulins in patients with diabetes. These data should enhance confidence in the use of the newer basal insulins in clinical practice by providing physiological context to real-world observations from heterogeneous patient populations.
Translating the Evidence into the Real World: New Perspectives and Old Challenges
Professor Kamlesh Khunti
Key Points
- Well-designed, real-world studies complement randomised controlled trials (RCT) by providing a fresh perspective on the use of basal insulins in the treatment of diabetes.
- Many different sources of real-world data are now available, such as claims databases, electronic medical records, patient registries, and health surveys.
- It is important to be aware of the strengths and limitations associated with each of these datasets.
- Improvements in data collection techniques and analytical methodologies have enabled real-world evidence studies to look beyond pre/post switch analyses and directly compare newer basal insulins in order to guide clinical practice.
Real-world studies offer a different perspective on the evaluation of therapies compared with RCT. While RCT determine the efficacy of therapies in a controlled setting (i.e. can it work?), real-world studies evaluate the effectiveness of therapies in a clinical setting (i.e. does it work?).13,14 Although RCT have proved essential in establishing the efficacy and safety of pharmaceutical products, translating the results of RCT to a broader patient population remains challenging, in part because RCT are conducted in a highly controlled environment that excludes large proportions of real-world patient populations.
Going Beyond Randomised Controlled Trials Data
Pragmatic trials and observational studies are a vital component in the continuum of clinical research. These real-world studies are conducted under usual-care settings and in broad patient populations to generate clinical data which complements that obtained from efficacy-focussed RCT, such as registration and long-term Phase III studies.15 As real-world studies can evaluate factors that impact treatment effectiveness in real-life, they provide a means to answer questions that cannot be addressed by RCT. Recent technological advances have led to improvements in analysis techniques for real-world studies, such as approaches for accounting for confounders and minimising bias.15
Interest in real-world studies and the evidence they can generate is growing among scientific, regulatory, and payer communities in both the USA and Europe. Initiatives to standardise the collection and analysis of real-world data include the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) real-world evidence framework for pharmaceutical industries, the European Medicines Agency’s (EMA) Patient Registry Initiative, and the US Food and Drug Administration’s (FDA) policy on the use of real-world evidence.16-18 Real-world data can now be obtained from a diverse array of sources such as claims databases, electronic medical records, patient registries, and health surveys, and can be collected prospectively or retrospectively (Figure 3).19,20 These data sources typically have broader eligibility criteria than RCT, and may be more reflective of the general patient population and clinical practice. While real-world studies can be utilised to examine clinical endpoints and quality of life measures, these studies also offer a means to investigate factors such as treatment adherence and persistence, psychosocial factors, healthcare utilisation, and financial burden on patients and healthcare systems.
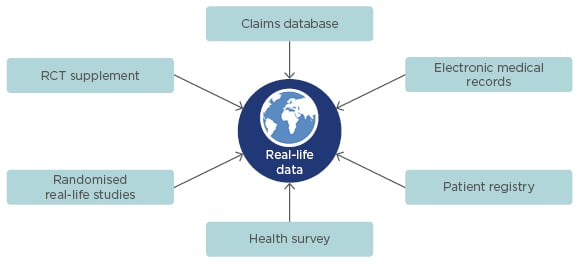
Figure 3: Sources of real-world data.18–20
RCT: randomised controlled trial.
What Does Real-World Evidence Tell Us About Basal Insulins?
RCT provide important insights on the efficacy of basal insulins. Evidence from the pivotal EDITION and BEGIN Phase III programmes has demonstrated that Gla-300 and insulin degludec, respectively, are associated with robust HbA1c-lowering and a reduced risk of hypoglycaemia versus Gla-100.21,22 A trial-level meta-analysis of clinical outcomes from the EDITION programme demonstrated that the risk of ≥1 confirmed (<54 mg/dL) or severe hypoglycaemic events was lower with Gla-300 compared with Gla-100 at night (00:00–05:59 a.m.), and also at any time (24 hours).23 Matching analyses of the BEGIN programme showed that insulin degludec compared with Gla-100 was associated with a lower risk of ≥1 confirmed (<56 mg/dL) or severe hypoglycaemic events at night, but comparable risk at any time.23 However, many real-world patients are under-represented in these types of trials. An analysis of patients with Type 2 diabetes mellitus living in Scotland in 2008 was conducted to describe the proportions of individuals who would meet eligibility criteria for inclusion in landmark RCT of glycaemic control, including ACCORD, ADVANCE, and VADT. The study revealed that, of the patient population surveyed (N=180,590), <36% of individuals were found to meet the inclusion and exclusion criteria of the seven RCT evaluated.24
Evidence from observational studies suggests that there is a disparity between data generated from RCT and what happens in the real world. SOLVE, a 24-week observational study involving 10 countries, assessed the safety and effectiveness of initiating once-daily insulin detemir in routine clinical practice among patients with Type 2 diabetes mellitus treated with ≥1 oral antidiabetic drug (OAD).25 The study demonstrated that prior to insulin initiation, mean HbA1c levels ranged from 8.3% in China to 9.8% in Turkey and the UK. These data indicate that patients remain poorly controlled on OAD treatment for prolonged periods of time prior to basal insulin initiation.25 Further analyses of SOLVE demonstrated that patients remained at a relatively low basal insulin dose at study end; the mean daily insulin dose was 0.27 U/kg at Week 24.25 Treat-to-target trials often report higher insulin doses compared with those recorded in observational trials, such as SOLVE. In one such treat-to-target trial, insulin-naïve patients with Type 2 diabetes mellitus were titrated to receive insulin detemir or Gla-100 once daily. After 52 weeks, the mean daily insulin detemir dose (n=227) was 0.78 U/kg.26 These differences indicate that in the real world, basal insulin titration is sub-optimal. Further analyses of the SOLVE data evaluated the relationship between mean HbA1c and total insulin dose.
Observational studies are also providing valuable insights on glycaemic goal attainment and maintenance in the real world. In a retrospective analysis of electronic medical records from five European countries and the USA, glycaemic control and hypoglycaemia were evaluated in 40,627 insulin-naïve adults (≥30 years old) initiating basal insulin, with or without OAD. Patients who achieved an HbA1c target ≤7.0% 3 months after basal insulin initiation were more likely to be at HbA1c target at 24 months after basal insulin initiation (odds ratio: 3.70; 95% CI: 3.41–4.00).27 DUNE, a 12-week observational study, assessed individualised HbA1c target achievement and its association with symptomatic hypoglycaemia (occurrence/frequency) in 3,139 patients with Type 2 diabetes mellitus either newly (at time of enrolment) or recently (<12 months) initiated on basal insulin therapy.28 The study demonstrated that >25% of all study participants achieved their personalised, physician-set HbA1c target after 12 weeks of any basal insulin treatment, irrespective of whether the patient had been initiated at the time of enrolment or in the previous 12 months.28 Furthermore, approximately 20% of these patients achieved their target HbA1c without experiencing additional hypoglycaemia.
In addition to providing insights into the effectiveness of basal insulins, real-world studies also provide valuable information on the occurrence of adverse events. The frequency of hypoglycaemic events reported in real-world settings was recently compared with those reported in clinical trials in a pooled analysis of 30 studies (real-world evidence, n=11; RCT, n=19) conducted in patients with Type 1 or Type 2 diabetes mellitus who were treated with basal, premix, or basal-bolus insulin therapy.29 Patients with Type 1 diabetes mellitus reported rates of hypoglycaemia (confirmed, severe, and nocturnal) were consistently higher in real-world studies compared with RCT. In contrast, analyses from patients with Type 2 diabetes mellitus demonstrated a considerable overlap between real-world and RCT-recorded hypoglycaemia rates.29 Global hypoglycaemia rates among patients with Type 1 diabetes mellitus were assessed in the HAT study, a 6-month retrospective and 4-week prospective study conducted in 24 countries worldwide. Rates of overall hypoglycaemia were high and varied substantially between geographical regions; rates ranged from 17.5 events per patient-year in South East Asia (n=224) to 93.9 events per patient-year in Latin America (n=427).30 Further investigation of the factors affecting these geographical differences may help optimise future therapies and reduce the risk of hypoglycaemia.
Real-world data on the newer basal insulin analogues are beginning to emerge from recently completed and soon-to-complete studies, including EU-TREAT (insulin degludec) and DELIVER-2 (Gla-300).31,32 EU-TREAT was a European, retrospective, non-interventional chart review study in patients with Type 2 diabetes mellitus who switched their basal insulin regimen to insulin degludec at least 6 months prior to data collection.31 Analyses of the data revealed that insulin degludec was associated with a significant reduction from baseline in mean HbA1c (0.5%; p<0.001) that was sustained at 12 months (p<0.001).31 Rates of overall hypoglycaemia (0.49; 95% CI: 0.26–0.91), non-severe overall hypoglycaemia (0.51; 95% CI: 0.28–0.92), and non-severe nocturnal hypoglycaemia (0.09; 95% CI: 0.04–0.20) were significantly lower in the 12-month post-switch period versus the pre-switch periods.31 Taken together, these data indicate that switching patients to insulin degludec substantially improves glycaemic control and reduces the risk of hypoglycaemia in patients with Type 2 diabetes mellitus.
DELIVER-2, a retrospective, observational study in patients with Type 2 diabetes mellitus, collected data on patients switching to Gla-300 from their pre-existing basal insulin regimen.32 Propensity score matching (1:1 ratio) identified 947 patients who switched to Gla-300 and 947 patients who switched to other basal insulins. At 6 months, Gla-300 was associated with significant reductions from baseline in mean HbA1c (0.6%; p<0.01), that were consistent with reductions observed with other basal insulin regimens (0.5%).32 Of note, baseline adjusted mean hypoglycaemia event rates were 25% lower with Gla-300 versus other basal insulin regimens (0.67 versus 0.90 events per patient-year, respectively; p<0.01).32 Additional analyses of the DELIVER-2 data revealed that the hypoglycaemia event rate associated with hospital inpatient or emergency department visits was 48% lower with Gla-300 versus other basal insulin regimens, a reduction in healthcare resource which could potentially translate to total savings of up to $2,000 per patient per year.32 A series of prospective, randomised real-life studies have been initiated to further examine Gla-300 versus other basal insulin regimens in the real-world setting. The ongoing ACHIEVE, REACH, and REGAIN studies will evaluate HbA1c reduction, hypoglycaemia rates, persistence, patient reported outcomes, and resource utilisation among insulin-naïve patients treated with Gla-300 versus other basal insulins and will provide additional insights into the effectiveness of Gla-300.33-35
In summary, a growing body of real-world evidence is now being generated from numerous sources of data and utilising robust methodologies. These data are providing valuable insights regarding the use of basal insulin in the real world that will ultimately guide clinical practice.
Conclusion
Translating clinical evidence into practice and ensuring it is applicable to the diverse population of patients who are on or initiating insulin therapy remains a challenge in diabetes care. From time-in-range to hypoglycaemia risk, time of basal insulin initiation to treatment adherence, and from prescription to patient education, multiple factors may impact outcomes, patient quality of life, and treatment satisfaction and effectiveness. Innovations in therapeutic basal insulin have been a continuous driver of improving patient care, particularly in recent years. Intrinsic improvements in basal insulin products, as well as new diabetes-related technologies and the availability of robust real-world evidence, are, together, producing an integrated understanding of how the use of basal insulin analogues can be optimised for the benefit of our patients.








