Speakers: Ian J. Neeland,1 Roy Taylor2
1. Director, University Hospital Center for Cardiovascular Prevention, University Hospitals Cleveland Medical Center, Case Western Reserve University School of Medicine, Cleveland, Ohio, USA
2. Newcastle University, Magnetic Resonance Centre, Campus for Ageing and Vitality, Newcastle upon Tyne, UK
Disclosure: Neeland has received grant support from NIH/NIDDK, and Novo Nordisk; has served as a consultant for Merck, Boehringer Ingelheim/Lilly Alliance, and Nestlé Health Science; and has been a former scientific advisory board member for AMRA Medical. Taylor is a member of UK government (SACN) working group on low-carbohydrate diets; is the author of the book ‘Life Without Diabetes’; has received lecture fees from Novartis, Lilly Alliance, Janssen, and Nestlé Health Science; and has received research funding from Diabetes UK.
Acknowledgements: Writing assistance was provided by Eleanor Roberts, Beeline Science Communications Ltd, London, UK.
Disclaimer: The publication of this article was sponsored by Nestlé Nutrition Institute and supported by an educational grant from Nestlé Health Science. The opinions expressed in this article do not necessarily reflect those companies.
Citation: EMJ Diabet. 2021;9[Suppl 1]:2-10.
Meeting Summary
Visceral or intra-organ fat (VIF) accumulation may be a better indication of cardiovascular and metabolic disease risk than BMI. The link with increased disease risk may be through dysfunctional adiposity, leading to excess ectopic fat deposition and hence inflammatory and adipokine dysregulation, insulin resistance, and atherogenic dyslipidaemia. Hepatic fat accumulation can lead to increased overnight glucose and, especially, to increased hepatic fat export, which can cause pancreatic fat accumulation leading to β-cell dysfunction. The failure of insulin secretion used to be viewed as irreversible in people with Type 2 diabetes (T2D) but is now recognised to be reversible once the metabolic stress of fat inside the pancreas is removed. T2D is not a problem limited to people with obesity; the concept of a ‘personal fat threshold’ (PFT) sheds light on how excess fat can push not only a person with a high BMI to develop T2D, but this can also occur in someone with a normal BMI who gains excess weight and intra-organ accumulation but stays within their BMI category. Interventions for VIF accumulation, including non-pharmaceutical, pharmacological, and surgical approaches, should be tailored to the individual, with lifestyle interventions being key for all. Dietary means to achieve this include a low-energy diet, approximately 800 kcal/day, for a defined period, as part of a supervised, supported weight management programme. At the American Diabetes Association’s (ADA) virtual 81st Scientific Session, a symposium was presented wherein Ian J. Neeland, Cleveland, Ohio, USA, discussed the role of intra-organ fat in obesity pathogenesis and Roy Taylor, Newcastle upon Tyne, UK, highlighted how analysis of intra-hepatic and intra-pancreatic fat using MRI can lead to insights into practical therapy for achieving effective weight loss and remission of T2D. This article reviews their findings and how weight loss interventions may not only help reduce cardiovascular and metabolic disease risk, but also reverse T2D for some patients.
INTRODUCTION
Obesity is traditionally measured by BMI;1 however, more recent studies point to how adverse outcomes associated with being overweight, such as cardiovascular disease (CVD) and T2D, may be more associated with VIF accumulation and distribution.2,3 As such, dietary programs may need to assess not only simple weight loss, but how they affect body fat distribution.
At the ADA 81st Scientific Session, 2021, held virtually, a symposium was presented by Neeland and Taylor that highlighted the role of VIF in the pathogenesis of obesity and T2D along with weight management programmes that can help target VIF.
Visceral Fat and its Role in the Pathogenesis of Obesity and Comorbidities
Ian J. Neeland
Visceral and Ectopic Fat Versus BMI
Currently, approximately 40% of the adult population in the USA are considered obese.4 This is of concern as obesity is associated with lifetime incidence of CVD morbidity and mortality.5 The traditional way to identify at-risk adults has been via calculation of BMI;1 however, according to Neeland: “We know that the BMI–health outcomes relationship is heterogenous. Many patients with obesity never develop CVD or metabolic diseases, whereas others who are not obese will.”
Neeland suggested that one of the most important aspects in determining such disease risk was actually variation in VIF burden. By illustration, he presented the case of a mildly overweight (BMI: 26 kg/m2) 43-year-old male with average low-density lipoprotein (LDL) cholesterol (116 mg/dL) and blood pressure (135/65 mmHg). Body fat analysis via MRI and advanced lipid analysis revealed that his visceral adipose tissue was at the 95th percentile for his age/BMI;6 his liver fat fraction was 25% (upper normal range: <5.5%);7 his HbA1c was 6.0% (pre-diabetes: 5.7−6.5%);8 he had an increased concentration of small LDL particles associated with atherogenic dyslipidaemia9 and elevated triglycerides of 240 mg/dL (reference: <150).9 Despite being classed as only mildly overweight, these figures, Neeland explained, meant this male had an elevated CVD and T2D risk and was a classic case of someone who was “obese on the inside” (Figure 1).
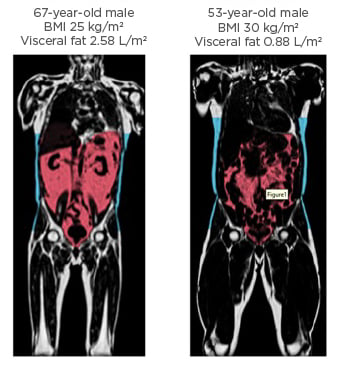
Figure 1: Extreme variation in abdominal fat distribution.
T1-weighted coronal neck-to-knee MRI images demonstrating variation in abdominal subcutaneous fat (blue) and visceral fat (red) between an individual with normal weight but high visceral fat (left) and an individual with obesity but lower visceral fat (right).
Images courtesy of AMRA Medical (Linköping, Sweden). Adapted from Neeland et al.3
What potentially underlies this is dysfunctional adiposity, which, according to Neeland, is “a pathologic response by adipose tissue to positive caloric balance in susceptible individuals that directly and indirectly contributes to cardiovascular and metabolic disease.” Dysfunctional adiposity is characterised by excess VIF deposition, inflammatory and adipokine dysregulation, insulin resistance, and atherogenic dyslipidaemia.2 This, said Neeland, “makes VIF a very attractive, modifiable target to track weight loss outcomes.”3
Ectopic fat accumulation starts when an imbalance between caloric intake and/or energy expenditure leads to a positive energy balance. Increased triglycerides deposited in adipose tissue can cause inflammation and, when adipose tissue can no longer expand, a spill-over of excess lipids into tissues that don’t usually contain them, such as the liver, pancreas, muscles, and heart. This, explained Neeland, can create inflammation and insulin resistance that can lead to adverse cardiometabolic outcomes.2
Indeed, in an investigation utilising data from the Dallas Heart Study, the risk factor most strongly predictive of T2D was visceral fat mass (odds ratio [OR]: 2.4; 1.6–3.7), with lower ORs for fasting glucose (OR: 1.9; 1.4–2.6), weight gain (OR: 1.3; 1.1–1.2), and systolic blood pressure (OR: 1.3; 1.1–1.5).2 Another study showed that with increasing quartiles of visceral adipose tissue came increasing incidence of CVD (hazard ratio: 1.21; 1.02–1.43).10
Interventions Targeting Visceral Obesity
Interventions for visceral obesity include non-pharmaceutical, pharmacological, and surgical approaches, and, Neeland highlighted, choice might depend on “the treatment intensity needed, the presence or absence of current disease, and whether or not the previous modality worked” (Figure 2). He further stressed that the key fundamental treatment for weight loss, as well as for VIF, stems from lifestyle interventions.
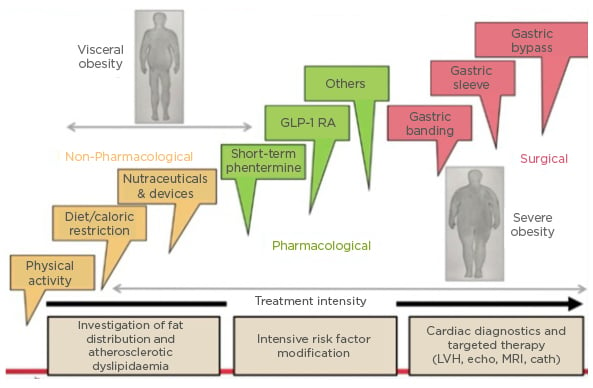
Figure 2: Interventions for (visceral) obesity.
Cath: cardiac catheterisation; echo: echocardiogram; GLP-1 RA: glucagon-like peptide 1 receptor agonists; LVH: left ventricular hypertrophy.
Adapted from Despres et al.11
Current USA weight management guidelines suggest a caloric-based dietary plan with an initial prescription of 1,200−1,500 kcal/day for females and 1,500−1,800 kcal/day for males, representing a 500−700 kcal/day energy deficit.12 A calorie-restricted diet can be based on patient preference, food types, and health status but the Mediterranean Diet is the diet with the most consistent and robust scientific support in reducing atherosclerotic CVD risk12 and the diet most suggested by Neeland to his patients to both lose weight and improve cardiovascular risk.
That lifestyle modification reduces VIF was illustrated by a study where 278 adults with obesity and/or dyslipidaemia were taught a multiple-daily lifestyle intervention that included either a low-fat or a Mediterranean/low-carbohydrate diet, with or without increased physical activity. After 18 months, the dietary modification cohort, regardless of physical activity, improved with regards to weight and waist circumference.13 Although the degree of weight loss wasn’t high (around 5% overall), pre- and post-imaging of VIF depots revealed that degree of fat decrease over time was much greater than weight change. Liver fat, for instance, went from 28% to 5%, which was, explained Neeland, “a much greater-fold decrease than one would expect if just monitoring weight loss and waist circumference.” What this showed, he continued, was that “even if the patient may not be losing generalised body weight…keep in mind that the VIF may be modified significantly, which has long-term beneficial outcomes.”13
Very Low Energy Diets
Myriad diets create confusion regarding what’s best, reported Neeland, with the ketogenic diet being the one he is asked about most. This is a restricted-carbohydrate, high-fat diet where the goal is 90% long- or medium-chain triglycerides in a 4:1 lipid to non-lipid ratio. Compared with usual care, one study showed that a ketogenic diet could significantly decrease several cardiometabolic risk factors, weight and lipid measurements, and improve glucose tolerance.14 However, Neeland posited that the success of this low-carbohydrate diet might actually be due to it being calorie restrictive. This is illustrated by a study that compared high- versus low-carbohydrate diets and found that the latter was approximately 500 kcal/day less. Weight loss was associated with longer diet duration and restriction of calorie intake but not with reduced carbohydrate content.15
Calorie-controlled restriction diets can be via either a total meal replacement (TMR) or partial meal replacement (PMR) regimen, typically in the form of shakes or bars. The OPTIWIN study involved 273 participants with a BMI of 30−55 kg/m2 and randomised to either the OPTIFAST programme (OP), as a TMR regimen, or a food-based regimen for 26 weeks. At 26 weeks, average weight loss in the OP group was 12.4±0.6% versus 6.0±0.6% in the food-based group (p<0.001). At 52 weeks, respective percentages compared to baseline were 10.5±0.6% and 5.5±0.6% (p<0.0001), with, importantly, greater fat mass loss in the OP group.16
In a study conducted by Optavia,17 participants followed a PMR regimen comprised of meal replacement products and one low-carb ‘lean and green’ meal and received support through regular coaching calls. When tested against the Metafast diet and a standard food-based diet, the PMR regimen resulted in greater weight loss and adherence, which was proposed to be attributed to the coaching calls.17 These studies highlight the potential utility of controlled, monitored, very low energy (VLE) diets.
Novel Non-pharmacological Weight Loss
Non-pharmacological approaches to weight loss include nutraceuticals and medical devices. Nutraceuticals are food supplements designed to promote weight loss by varying mechanisms. Some, such as green tea, inhibit, delay, or modify gastrointestinal nutrient absorption. Others act as a metabolic modulator, whey protein being one example, or appetite regulators, e.g., diacylglycerol, which helps increase fat metabolism. Finally, another category, exemplified by L-carnitine, increase energy metabolism.18
Whey protein stimulates secretion of incretin peptides, particularly glucagon-like peptide (GLP-1), insulin, and gut peptides, resulting in delayed or slowed gastric emptying.19 In the Zeus pilot study20 (N=26; mean BMI: 29.1 kg/m2), 10 g whey protein in the form of a microgel (WPM) was taken as a pre-meal shot, 15 minutes prior to consumption of a test meal. The WPM significantly augmented GLP-1 response,21 which, according to Neeland, shows that whey protein can act as a GLP-1 agonist and may help reduce glycaemic variability and increase weight loss.22,23 “With this understanding,” Neeland discussed, “things like WPM may be an inexpensive and low-risk way to induce GLP-1 agonism in metabolism to improve weight loss and reduce T2D risk.”
Mechanical devices, such as intra-gastric balloons, can be ingested or placed surgically and induce weight loss through increased satiety or alteration of absorption of food contents. While there are several endoscopic weight loss devices available, Neeland advised caution as implantation needs to be carried out under sedation and adverse events, though rare, have included bowel perforation, bleeding, obstruction, device migration, and pulmonary aspiration.24
Non-mechanical, expanding, non-caloric ingestible devices essentially produce satiety. One such is Gelesis 100/Plenity® (Gelesis, Boston, Massachusetts, USA), a prescription capsule that contains hydrogel particles that expand in the stomach after ingestion. The particles transit in the gastrointestinal tract, disintegrate, and are excreted. Data show weight loss of approximately 3−5% compared with standard care.25 The Epitomee® device (not currently approved; Epitomee Medical, Caesarea, Israel) is a biodegradable, encapsulated, shape-shifting device made of absorbent polymers that expands in the stomach to create a super absorbent, pH-sensitive gel structure that mimics a large, solid food mass. The device disintegrates into small particles within 30 minutes, minimising the risk for gastric obstruction. In a single-arm pilot study, 52 participants (baseline BMI: 27−40 kg/m2) took the capsule twice daily for 12 weeks and showed weight loss of 3−4% on average.26 Though weight loss was modest with both devices, Neeland pointed out that “the risk for these devices is relatively low so they might be a good choice for a patient who wants to increase their satiety not using pharmacological means.”
To conclude, Neeland highlighted that “while at a population level standpoint BMI is related to adverse cardiometabolic outcomes, for personalised medicine approaches, we need to go beyond BMI to identify those at highest risk who would benefit from therapies in order to decrease any adverse events that might occur.”
Magnetic Resonance Insights to Practical Therapy: Achieving Effective Weight Loss and Remission of Type 2 Diabetes
Roy Taylor
The ‘Personal Fat Threshold’
Hepatic insulin sensitivity is related to the amount of fat in the liver and overnight fasting glucose production, which is found to be higher in people with T2D.27,28 As the liver is responsible for distributing fat throughout the body, Taylor highlighted how, if there is excess fat build-up in the liver because of excess calories, “the speed of that process is increased so excess fat…will silt up in ectopic sites including the pancreas.”27 Excess fat here can lead to metabolic stress in β-cells, causing them to go into “survival mode”, de-differentiate, and lose their specialist function.27
Historically, T2D development has been associated with obesity; however, in an analysis of data from the UK Prospective Diabetes Study (n=5,102),29 Taylor pointed out how BMI incidence for people with T2D was only slightly skewed from what is considered healthy (<25.0 kg/m2), to a median BMI of 28 kg/m2, with only a relatively minor percentage having a BMI >35 kg/m2.30 In the Nurses’ Health Study, which followed females from early to later life, the relative risk (RR) for developing T2D in people with a BMI <23 kg/m2 compared to those with a BMI ≥35 kg/m2 was very high (RR: 38.8; 95% confidence interval [CI]: 31.9−47.2). However, there was also a notable increase in RR for those with a BMI of 25.0−29.9 kg/m2 (RR: 7.59; 95% CI: 6.27−9.19) or even of 23.0−24.9 kg/m2 (RR: 2.67; 95% CI: 2.13−3.24).31
These findings challenge the idea of a tight relationship between BMI-determined obesity alone being the cause of T2D. Taylor discussed how people should be thinking more about the concept of a PFT and how when a person is carrying more fat than their body has metabolically coped with in the past can lead to crossing a PFT into the zone where T2D develops (Figure 3A, where the dotted line is each person’s PFT). For instance, a male who was underweight aged 21 years who gained weight in middle age but was still not considered overweight at this time could in fact be carrying as much excess fat on his body compared to his 21-year-old self as someone who went from being overweight aged 21 years to being obese in middle age.30
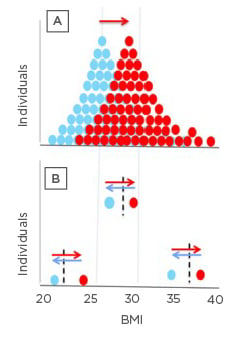
Figure 3: Personal fat threshold.
Representative frequency distribution of BMI for people with Type 2 diabetes before (blue) and after (red) they gained weight. The frequency distribution indicates a higher prevalence of obesity in this cohort who gained weight. However, looking at individuals (B), one started out as obese, one overweight, and one normal weight; all lost 15 kg weight (blue arrows) and returned to normal glucose tolerance once they’d crossed back over their personal fat threshold, even though their BMI category remained the same.
Adapted from Taylor, Holman.30
In line with this concept, Taylor presented data showing that a 15 kg weight loss in a person of any weight who develops T2D could restore normal health, and stressed how useful it was to educate patients about their PFT and explain how it is β-cell susceptibility to excess fat that determines T2D risk. This would enable patients that develop T2D, but without obesity, to understand why all people with diabetes need to lose weight to have a chance of remission.
Type 2 Diabetes and Very Low Energy Diets
A number of years ago, Taylor and colleagues postulated that a negative calorie balance should decrease hepatic fat levels, improve pancreatic insulin response, and normalise overnight blood sugar. In the Counterpoint study, 11 people with T2D (not on insulin; <4 years duration; BMI: 33.5±1.2 kg/m2) were given a liquid-based diet (600–800 kcal/day) for 8 weeks. Within the first 7 days, fasting plasma glucose (FPG) normalised (5.9±0.4 mmol/L down from 9.2±0.4 mmol/L) and hepatic triacylglycerol content reduced to 2.9±0.2% from a baseline mean of 12.8±2.4%. By Week 8, FPG remained at normal levels and hepatic triacylglycerol content was down to 2.9±0.2%. Critically, there was a gradual fall in pancreatic fat and a return of normal first-phase insulin response.32 These results, emphasised Taylor, held up the hypothesis of positive effects of substantial weight loss on T2D indices.
Of note in the Counterpoint study, only people who had T2D for <4 years were included. The CounterBALANCE study included 30 people with T2D, ranging in duration from diagnosis from 0.5−23 years, who followed a similar liquid diet (624−700 kcal/day) for 8 weeks then a 2-week stepped introduction to solid food and a 6-month structured, individualised weight-maintenance phase.33 In the diet ‘responders’ (FPG <7.0 mmol/L) acute insulin response returned to normal at 10 weeks and remained so at 6 months.33 This was important, noted Taylor, as it showed there was no inevitable progressive β-cell failure.
In the ‘non-responders’ at baseline, acute insulin response was almost zero, with little change over the study period. Taylor highlighted that while responders had an average diabetes duration of 3.8±3.3 years, the non-responders average was 9.8±1.6 years, indicating that time with diabetes may impact how recoverable β-cells are; however, he urged, this doesn’t mean that those with a long duration of T2D will not respond to a VLE diet. In fact, Taylor described two patients from his clinical practice whom after 23 years with T2D achieved normal glycaemia and cessation of all anti-diabetes medications with a VLE diet.
With the idea that substantial weight loss could help reverse diabetes, Taylor and colleagues carried out the DiRECT trial to test the feasibility of a VLE diet in routine primary care. Patients with T2D of <6 years duration were randomised to an intervention (n=64) or control (n=26) group. The intervention group stopped all anti-diabetic medications on Day 1 of the programme, which consisted of an 825−853 kcal/day liquid formula diet for 12−20 weeks, followed by a 2−6 week food reintroduction phase then ongoing weight-maintenance support. The control group received usual diabetes standard of care.34
At 5 months, while both responders and non-responders lost weight, in responders only, decreases were shown in FPG and HbA1c (to <5.9%), which was maintained at 12 months without medication. In all programme participants, liver fat fell, from approximately 15% to approximately 3%, with pancreas fat falling to a lesser extent. Acute insulin response increased significantly in the responders.34
Another study included participants from DiRECT matched in age, sex, and weight with normoglycaemic controls. This utilised MRI to show that the pancreas in those with T2D was of significantly lower volume than in the normoglycaemic control group prior to the intervention. At 24 months, the pancreas volume of the responders increased to near-normal levels.35 “This gross change in whole pancreas,” stressed Taylor, “really emphasises the nature of remission: it is a return to the normal state, not just well-controlled T2D.”
Also of note in the DiRECT study was how the intervention affected blood pressure. One analysis included a subset of 78 patients in the intervention group (n=143) that were taking antihypertensive medications prior to the start of the study but in whom these were completely (85%) or partially (5%) discontinued on Day 1. By Week 20, SBP decreased approximately 10 mmHg in all sub-groups regardless of whether they had a history of hypertension or discontinued or remained on their hypertension medications.36
Concerns are often expressed about the safety of a VLE diet; however, Taylor pointed out that of the DiRECT participants who were followed over 24 months, 19 participants (13%) in the control group (n=149) experienced 25 serious adverse events (SAEs), but there were only 15 SAEs in 11 participants (7%) in the intervention group (n=149). SAEs in the control group included one fatal myocardial infarction, two cerebrovascular disorders, one aortic aneurysm, five weight-related cancers, and one toe amputation; in the intervention group there were no fatalities, no amputations, and no cancers.37 “This is nothing to do with the cause of cancer though,” pointed out Taylor, “but with the rate of progression and presentation as insulin is a major factor in promoting tumour growth and inhibiting apoptosis.”
During the Counterpoint study, Taylor discussed how he and his team realised that for many of the participants, success was determined by how on-board and involved their spouse or partner was; hence, it is vital to include a person’s family in a patient’s dietary goals. Another challenge in the earlier studies was that, according to Taylor, while the diet was easier than people expected, the change back to eating solid food was harder. However, Taylor emphasised that the solid food reintroduction period can serve as a ‘clean slate’ opportunity, whereby specific behavioural and dietary modification education can be provided on what to eat for the long-term to avoid weight regain.
Another factor to be taken into account is how stressful life events can lead to poor weight management and weight gain. In the DiRECT study, participants were offered ‘rescue plans’ whereby if they gained ≥2 kg they would go back onto PMR for 2−4 weeks and if they gained >4 kg they could return to TMR for 4 weeks, followed by 4 weeks food introduction. “In the end,” discussed Taylor, “there was no difference in outcomes in those who required rescue plans and those who didn’t.”37 This was, he suggested, because “weight regain is not a failure or a disaster, it just calls for focused attention when people can re-engage with dietary restriction.”
Taylor concluded that at T2D diagnosis, people must be offered a choice of conventional medication therapy or supervised weight loss and potential freedom from diabetes. For this, personal planning, including family members, is essential, and the target should be 15 kg weight loss.
SUMMARY
Overall, both Neeland and Taylor emphasised the need to look beyond BMI and investigate indices of body fat distribution to understand a patient’s risk for developing cardiovascular and metabolic diseases. In those where this is an issue, there are a variety of interventions, with lifestyle modifications being key.
Caloric restriction can successfully reduce VIF and reverse T2D, including β-cell dysfunction; however, weight loss is best carried out in a supportive setting with weight maintenance and ‘rescue’ plans in place once the VLE diet part of a programme is finished.








