Abstract
Gestational diabetes mellitus (GDM) is a major public health problem with various complexities involved in its diagnosis. Traditionally an oral glucose tolerance test is used for the diagnosis of GDM, however the measurement of plasma glucose values both after fasting and the glucose challenge test has certain shortcomings, especially during pregnancy. The American Diabetes Association (ADA) in 2010 and the World Health Organization (WHO) in 2011 have accepted glycated haemoglobin (HbA1c) as a tool for diagnosing diabetes mellitus, however it is not currently recommended as a diagnostic tool for GDM. The estimation of HbA1c levels is likely to be more acceptable to pregnant women, as a single non-fasting blood sample is required for this investigation. Although various studies have shown different HbA1c cut-off values representing the best equilibrium between sensitivity and specificity for GDM, most of them conclude that an HbA1c level of >5.95% can be used to diagnose GDM in pregnant women with high specificity. This article reviews the present role and future place of measuring HbA1c levels in the diagnosis of GDM.
INTRODUCTION
Gestational diabetes mellitus (GDM) is diabetes diagnosed in the second or third trimester of pregnancy that is not clearly either Type 1 or Type 2 diabetes. Women with diabetes diagnosed in the first trimester would be classified as having pre-existing undiagnosed Type 2 diabetes mellitus.1 The early diagnosis and treatment of GDM is of utmost importance as it can put both the pregnant woman and her fetus at risk of various complications such as pre-eclampsia, polyhydramnios, preterm labour, caesarean delivery, shoulder dystocia, birth injury, and neonatal hyperbilirubinemia. Also, women with GDM and their newborns have a significantly increased risk for the subsequent development of impaired glucose tolerance, metabolic syndrome, and overt diabetes.2 The landmark HAPO3 study of approximately 25,000 pregnant women demonstrated a strong and graded relationship between maternal glycaemia and adverse pregnancy outcomes, and that the majority of clinical complications caused by GDM can be prevented by early diagnosis and adequate control of blood glucose levels.
PREVALENCE OF GESTATIONAL DIABETES MELLITUS AND DIAGNOSTIC CRITERIA
The prevalence of GDM varies from 2.4–21.0% of all pregnancies depending upon the diagnostic criteria used to define GDM.4-8 The prevalence of GDM in the UK, USA, and among European countries was estimated to be 5%, 3–7%, and 2–6%, respectively.9,10 In a random survey performed in various cities in India from 2002–2003, an overall GDM prevalence of 16.55% was observed.7 In contrast the prevalence of GDM reported from the northern part of India varies from 3.8–13.9%.11-13 The varying prevalence rate across different studies highlights the fact that there is no uniform or best way to diagnose GDM throughout the world. The oral glucose tolerance test (OGTT) is the gold standard for diagnosing GDM and various associations across the globe recommend different glucose values for diagnosing GDM with the OGTT.14 The test most commonly used to diagnose GDM is recommended by the American Diabetes Association (ADA) and is a 3-hour, 100 gOGTT. GDM is diagnosed if two or more plasma glucose levels are greater than or equal to the following values: fasting glucose concentration of 95 mg/dL, 1-hour glucose concentration of 180 mg/dL, 2-hour glucose concentration of 155 mg/dL, or 3-hour glucose concentration of 140 mg/dL.15 Based on the recommendations given by the International Association of the Diabetes and Pregnancy Study Groups (IADPSG), the ADA also recommends the use of a 2-hour 75 g OGTT with glucose thresholds of 92 mg/dL, 180 mg/dL, and 153 mg/dL for fasting, 1-hour, and 2-hour values, respectively. A diagnosis of GDM is made when any of the above-mentioned plasma glucose values are met or exceeded.16,17 The World Health Organization (WHO) diagnostic criteria are based on a 2-hour 75 g OGTT and GDM is diagnosed if either the fasting glucose is >126 mg/dL or the 2-hour glucose is >140 mg/dL.18 The fasting value suggested by the WHO is similar to the diagnostic criteria for diabetes mellitus in non-pregnant individuals and the 2-hour value is similar to the diagnostic criteria for impaired glucose tolerance in non-pregnant individuals. The conflicting recommendations underscore the important point that there are insufficient data to strongly demonstrate the superiority of one strategy over the other and that further research is needed to resolve these uncertainties.
SHORTCOMINGS OF PLASMA GLUCOSE AND THE ORAL GLUCOSE TOLERANCE TEST FOR THE DIAGNOSIS OF GESTATIONAL DIABETES MELLITUS
OGTT, though it is the gold standard, is a cumbersome procedure for the participant as well as healthcare providers and has several limitations. It requires the participant to be in a fasting state, at least 2 hours for sample collections, and a minimum of two blood samples to be taken. The time required and the number of samples to be collected can be higher depending upon the criteria followed. The other limitations include uncertainty of fasting state, poor reproducibility of 2-hour glucose, poor concordance between the fasting plasma glucose (FPG) and 2-hour plasma glucose, and that a few days or weeks of change of lifestyle, including dieting or increased exercise, significantly affect both FPG and OGTT. Both the ADA in 2010 and the WHO in 2011 have accepted glycated haemoglobin (HbA1c) as a diagnostic tool for diagnosing diabetes mellitus. In comparison with glucose measurements, the use of HbA1c as a diagnostic test has advantages including: convenience, as fasting is not needed for assessment, less day-to-day variability, and greater pre-analytical stability with international standardisation not inferior to a glucose assay. The intra-individual coefficient of variation for measuring FPG has been found to be 6.4–11.4% and 14.3–16.7% for the measurement of 2-hour plasma glucose.19,20 Furthermore, the inter and intra-individual variabilities of fasting glucose and 2-hour glucose are higher during pregnancy compared with the non-pregnant state.21 Compared with this, HbA1c measurement has excellent reliability with an intra-individual coefficient of variation of 4.2% over the short-term in patients with diabetes and 1.9% over the long-term in persons without diabetes.20,22 However, HbA1c measurement also has certain limitations, such as being more costly than plasma glucose. Some haemoglobin traits such as HbS, HbC, and HbF interfere with some HbA1c assays and any condition that changes red cell turnover, such as haemolytic anaemia, chronic malaria, major blood loss, or blood transfusions, will lead to spurious HbA1c results and a lack of concordance between fasting/ 2-hour plasma glucose and HbA1c. HbA1c was not standardised in the past and this was only achieved after significant international effort. As it stands, current guidelines do not adequately reflect the accuracy of HbA1c measurements available across the nation at the current level of standardisation. Although HbA1c is now recommended for the diagnosis of diabetes, there are no recommendations available for the use of HbA1c as a diagnostic tool for GDM.
UTILITY OF GLYCATED HAEMOGLOBIN FOR THE DIAGNOSIS OF GESTATIONAL DIABETES MELLITUS
HbA1c is a form of haemoglobin used primarily to identify the average plasma glucose concentration over a prolonged period of time. It is formed in a non-enzymatic pathway by haemoglobin’s normal exposure to high plasma levels of glucose.23 Hyperglycaemia induces excessive production of the early glycation products as an acute reversible change. Glucose rapidly attaches to the amino groups of the proteins through the non-enzymatic process of nucleophilic addition to form Schiff base adducts. These adducts reach equilibrium levels that are proportional to the blood glucose concentration within hours and subsequently undergo rearrangement to form more stable glycation products. One of the proteins glycated in this way is HbA1c. Once a haemoglobin molecule gets glycated, a build-up of HbA1c within the red blood cells (RBCs) reflects the average level of glucose to which the cell has been exposed during its life cycle.24 The HbA1c level is proportional to the average blood glucose concentration over the previous 4 weeks–3 months. It is important to note that HbA1c measurements are affected by the lifespan of the RBCs and the laboratory method used. HbA1c levels are routinely measured by high performance liquid chromatography (HPLC) methods. If it is assumed that the rate of glycation reaction is proportional to haemoglobin concentration along with the access of the side chain amino acid of haemoglobin to glucose and that the lifespan of the RBCs is constant, then HbA1c would be a suitable indicator of plasma glucose concentration during the lifespan of RBCs. Measurement of HbA1c is based on the presence of normal haemoglobin. Haemoglobinopathies can affect the accuracy of the test by interfering with thenormal process of glycation of haemoglobin A to A1c and making the RBCs more prone to haemolysis, thereby decreasing the time for glycation to occur and producing a false HbA1c result.25
HbA1c levels significantly decreased early in pregnancy and further decreased in late pregnancy compared to age-matched non-pregnant women. The normal range of HbA1c levels was found to be 4.7–6.3% in non-pregnant women, 4.5–5.7% in early pregnancy, and 4.4–5.6% in late pregnancy.26 Another study also observed that HbA1c levels were lower in pregnant women than in control women.27 This result is likely to be because of the normal decrease in FPG in early pregnancy, which is caused by glucose being diverted to the developing fetus. It is sustained throughout the pregnancy by increasing insulin resistance, which is most prominent in the third trimester of pregnancy. Additionally, the life span of RBCs is reduced during pregnancy and this results in shorter exposure times to plasma glucose and reduced glycation for new RBCs.28,29 As HbA1c represents the mean plasma glucose in the preceding 3–4 months, it is widely believed that it is not suitable for the diagnosis of GDM because of the long time required for changes in HbA1c levels. Although glycation of haemoglobin occurs over the entire 120-day life span of RBCs, it has been shown that the mean plasma glucose of the last 1 month contributes to 50% of the final result.30 Therefore, HbA1c may have a role in the screening and management of GDM, especially if lower cut-off values than those recommended for the diagnosis of diabetes mellitus in non-pregnant adults are used, and these values need to be validated by long-term studies.
The use of HbA1c for monitoring the degree of control of glucose metabolism in diabetic patients was first proposed in 1976. Initial studies during 1980–1990 that evaluated HbA1c as a possible screening test for GDM did not favour HbA1c as suitable. Frisoli et al.31 found that the mean HbA1c was higher in pregnancy and concluded that it was unreliable for GDM screening. Artal et al.32 reported that the high incidence of false negatives and false positives made HbA1c inadequate for GDM screening. Odsaeter et al.33 in their study on HbA1c as a screening test for GDM in pregnant women with polycystic ovarian syndrome could not discriminate between GDM and normal glucose tolerance throughout pregnancy. However, first trimester HbA1c was statistically significantly associated with pre-eclampsia. Both HbA1c and GDM by WHO criteria in the first trimester, but not by IADPSG criteria, were negatively associated with birthweight. Soumya et al.34 found a mean HbA1c level in women with GDM of 6.2±0.6%, whereas it was 5.4±0.5% in those with normoglycaemia. The receiver operating characteristic (ROC) curve of HbA1c for diagnosis of GDM had an area under the ROC curve (AUC) of 0.826. They found that women with GDM had a higher incidence of pregnancy-related complications compared with normoglycaemic women. An HbA1c ROC curve cut-off of 5.3% had a sensitivity of 95.6% and a specificity of 51.6% for the diagnosis of GDM. OGTT would have been avoided in approximately half of antenatal women whilst missing 5% of the women. However, those with an abnormal HbA1c level required a confirmatory OGTT, as 50% of normoglycaemic women would be misclassified as having GDM by this approach. On repeat testing postpartum, 2 out of 45 women (4.4%) had overt diabetes mellitus, whereas 5 (11.1%) had impaired glucose tolerance. The study concluded that although HbA1c cannot replace OGTT in the diagnosis of GDM, it can be used as a screening.
The various other studies published later used HbA1c level as a diagnostic tool for GDM and their recommendations of its utility in the diagnosis of GDM over OGTT are summarised in Table 1. Aggarwal et al.35 studied 442 pregnant women assessed for GDM by HbA1c. Two thresholds were used to rule in or rule out GDM which was confirmed by WHO criteria of 75 g OGTT. The AUC of HbA1c to detect GDM was 0.54 (95% confidence interval [CI]: 0.48–0.61). Using a value of <5.5% to rule out GDM a sensitivity of 82.1% was achieved, with 16.7% women below the threshold being false negatives (negative predictive value 83.3%). Using a threshold of HbA1c ≥7.5% to rule in GDM the specificity was 95.8% with 71.4% patients over the threshold being false positives (positive predictive value [PPV] 28.6%). They concluded that HbA1c would eliminate the need for an OGTT in 25.1% of patients. At any HBA1c threshold with an acceptable sensitivity the false positive rate remained high, resulting in too many healthy women proceeding to the confirmatory OGTT.
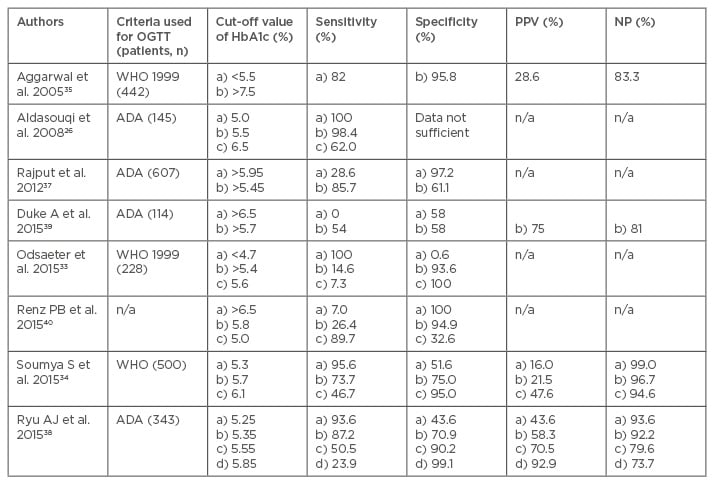
Table 1: Studies concerning utility of glycated haemoglobin in the diagnosis of gestational diabetes mellitus.
ADA: American Diabetes Association; HbA1c: glycated haemoglobin; NPV: negative predictive value; OGTT: oral glucose tolerance test; PPV: positive predictive value; WHO: World Health Organization.
Aldasouqi et al.26 in their retrospective study of 145 eligible patients found GDM in 124 patients with OGTT. The percentages of patients with HbA1c values (reference range of 4.8–6.0%) equal to or above sequential cut-off values of 5.0%, 5.5%, 6.0%, 6.5%, and 7.0% (i.e. sensitivity values) were 100%, 98.4%, 87.1%, 62.9%, and 39.5%, respectively. The mean HbA1c of the patients with GDM was 6.9±0.8% compared with 6.4±0.6% for those without GDM. At an arbitrary cut-off value of 6.0% (the upper limit of normal), HbA1c would have picked up 87.1% of patients with GDM. This study suggested that HbA1c is a reasonably sensitive screening measure for GDM in this high-risk population. Balaji et al.36 established the normal mean HbA1c values in Asian-Indian pregnant women as 5.36±0.36%. They also found that the mean HbA1c level in women with GDM at diagnosis during different trimesters was 6%.
In our study of 607 patients in 2012, the values of HbA1c ranged from 4.0–6.1%. The mean standard deviation HbA1c value in women with GDM was 5.73±0.34%, while it was 5.34±0.35% in women without GDM. The difference in the two HbA1c values was found to be statistically significant. The AUC of HbA1c level to detect GDM was 0.805 (95% CI: 0.687–0.923). It was observed that an HbA1c cut-off value of 5.95% had a sensitivity of 28.6% and a specificity of 97.2% in diagnosing GDM. An HbA1c cut-off value of 5.45% had a sensitivity of 85.7% and a specificity of 61.1% in diagnosing GDM. Using HbA1c as the initial test, if the level of HbA1c is ≥5.95%, then the woman can be labelled as having GDM. If the HbA1c level is <5.45%, then she may be labelled as not having GDM. For women with an HbA1c level lying between 5.45% and 5.95% an OGTT should be performed to correctly identify women with GDM. Using this methodology 85.7% of the GDM cases would have been detected and only 2.8% of women would have been wrongly labelled as having GDM. This methodology would also have obviated the need for an OGTT in 61.8% of women as observed in our study. A ROC curve was also drawn to determine the sensitivity and specificity of HbA1c in detecting GDM as defined by the IADPSG criteria. The AUC of HbA1c level to detect GDM was 0.683 (95% CI: 0.601–0.765). It was observed that an HbA1c cut-off value of 5.95% had a sensitivity of 11.9% and a specificity of 97.1% in diagnosing GDM. An HbA1c cut-off value of 5.25% had a sensitivity of 83.1% and a specificity of 40.5% in diagnosing GDM.37 However with the new IADPSG criteria a woman may be labelled as without GDM only when HbA1c is <5.25%, in contrast to the ADA criteria where an HbA1c level of <5.45% is required to identify pregnant women without GDM. For women with an HbA1c level lying between 5.25% and 5.95%, an OGTT should be performed to correctly identify women with GDM. Using this methodology 83.1% of the GDM cases would have been detected, only 2.9% of women would have been wrongly labelled as having GDM, and this methodology would have obviated the need for an OGTT in 39.66% of women. In this study we showed that irrespective of the criteria used for the diagnosis of GDM, an HbA1c level of >5.95% has a specificity of >97% in identifying cases of GDM.37
In another study by Ryu et al.,38 an HbA1c cut-off value ≥5.35% had the highest Youden index (0.581) and a high sensitivity (87.2%) in detecting GDM. However, the specificity was low (70.9%) and the false positive rate was 29.1%. The AUC for HbA1c detection of GDM was 0.852 (95% CI: 0.808–0.897). An HbA1c cut-off value ≥5.35% had maximal points on the Youden index (0.581). The sensitivity was 87.2% and the specificity was 70.9% for diagnosing GDM. A threshold value of ≥5.35% indicated that 163 patients had GDM and 68 (41.7%) were false positives. The PPV was 58.3% at this threshold value. In practical terms this means identification of 87.2% of the diseased patients, and that 29.1% of patients without GDM would be misdiagnosed. Conversely, 12.8% of the GDM patients would be missed. The study concluded that despite the positive correlations between HbA1c and GDM diagnosis, the utility of the HbA1c level as a diagnostic test for GDM remains controversial and despite improved standardisation and wide availability the HbA1c cannot replace OGTT for the diagnosis of GDM.
Duke et al.39 found the overall concordance between the OGTT and HbA1c for the diagnosis of diabetes, prediabetes, or normal glucose tolerance was only 54%. Gravidity, the 2-hour glucose level on the OGTT during pregnancy, and the third trimester HbA1c level predicted discordance between the postpartum OGTT and HbA1c. The sensitivity of an HbA1c level ≥6.5% for the diagnosis of diabetes alone was 0% and the specificity was 58%. The sensitivity of an HbA1c level ≥5.7% for the diagnosis of prediabetes and diabetes was 54% and the specificity was 58%, with a PPV of 75% and a negative predictive value of 81%. The study concluded that utilisation of the HbA1c test in postpartum women was not reliable for the exclusion of diabetes.
In a recent study by Renz et al.,40 a HbA1c cut-off point of 6.5% for diagnosing GDM presented 100% specificity but very low sensitivity (7.0%); HbA1c 5.8% showed adequate specificity in diagnosing GDM (94.9%) but a low sensitivity (26.4%). An HbA1c value of 5.0% presented adequate sensitivity (89.7%) but low specificity (32.6%). For women with an HbA1c value of 5.8%, the positive and negative likelihood ratios were 5.14 (95% CI: 2.49–10.63) and 0.78 (0.68–0.88), respectively. The study concluded that a HbA1c cut-off point of 5.8% could eliminate the need for the unpleasant and laborious OGTT tests in almost one-third of cases.
Various studies also analysed the pregnancy outcome alongside HbA1c values. Capula et al.41 confirmed that the proportion of pregnancies presenting negative outcomes increased progressively with increasing HbA1c levels, from 6.2% for HbA1c levels <5% to 18.3% for HbA1c levels from 5.0–5.3%, to 37.8% in patients with HbA1c levels from 5.4–5.6%, to 56.2% for HbA1c levels >5.6%. ROC analysis showed that HbA1c levels at diagnosis and before delivery were a good predictor of an adverse pregnancy outcome.
FUTURE ROLE OF GLYCATED HAEMOGLOBIN IN THE DIAGNOSIS OF GESTATIONAL DIABETES MELLITUS
The ongoing diabesity epidemic has led to an increasing number of women developing dysglycaemia during pregnancy and therefore it is reasonable to develop testing strategies which can identify such high-risk women correctly and promptly. Sensitivity is the proportion of true positives that are correctly identified by the test whereas specificity is the proportion of true negatives that are correctly identified by the test. A diagnostic test with higher specificity will have fewer false positives as compared to another test with lower specificity. For diagnosing GDM, it is preferable to use an HbA1c value with a higher specificity so that fewer patients with a false positive value are detected which would otherwise cause a false alarm, both to the patient and the treating physician. HbA1c levels >5.95% have an excellent specificity in confirming the diagnosis of GDM. Although an HbA1c cut-off value of 5.3–5.5% presents the best equilibrium between sensitivity and specificity, the sensitivity values at these cut-off points are not enough to rule out GDM. Multicentre multinational studies are required to settle this issue. Using the HbA1c level as the initial test, the patient can be labelled as having GDM if the level of HbA1c is >5.95%.
CONCLUSION
To conclude, although at the present time the HbA1c level cannot replace OGTT for the diagnosis of GDM, it can be used in combination with OGTT to obviate the need for OGTT in a large number of pregnant women. This is beneficial as the OGTT is more time-consuming, cumbersome, and has a marked intra-individual coefficient of variation. Using the HbA1c level is also likely to be more acceptable to pregnant women as a single non-fasting blood sample is required for this investigation. The results of the various studies from different parts of the world suggest that there is a need to define population-specific values for HbA1c levels rather than using one value to rule out GDM in all pregnant women. Also, irrespective of whether the ADA criteria or the recently proposed IADPSG criteria are used to diagnose GDM, an HbA1c level >5.95% can be used to diagnose GDM in pregnant women with a high specificity. The utility of measuring HbA1c levels for the diagnosis of GDM is evolving at the present time and the current literature definitely provides a framework for future research regarding its use in the diagnosis of GDM.








