Abstract
The majority of patients with Type 2 diabetes require insulin therapy for treating hyperglycaemia. There are several regimens available for insulin initiation and maintenance. Insulin analogues have been developed to mimic normal physiology as closely as possible. Biphasic analogues can target both fasting and postprandial hyperglycaemia, with the added advantage of being premixed and thus convenient for the patient. A practical and feasible option is to initiate insulin with one or more biphasic preparations at mealtimes, thus providing both basal and prandial coverage. Individual titration of dose and frequency of daily injections with biphasic insulin preparations has the potential for improving glycaemic control with a high degree of patient acceptance. Drawbacks include a more rigid regimen, a relative lack of flexibility, and a somewhat higher degree of glycaemic variability and hypoglycaemia when compared to multiple daily basal-bolus injections. Awareness of the advantages and limitations of biphasic insulin analogues can assist clinicians in their appropriate use for the treatment of patients with Type 2 diabetes.
INTRODUCTION: GLYCAEMIC CONTROL IN TYPE 2 DIABETES
Optimal management of hyperglycaemia is a key factor in preventing the long-term complications of diabetes. Although Type 2 diabetes (T2D) is an inexorably progressive disease, treatment options have expanded greatly. The consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes (ADA/EASD)1 advises lifestyle modification, including dietary changes and physical activity, as the cornerstone of therapy for glucose control. Metformin is recommended as the first-line pharmacotherapy if glycaemic targets are not achieved. Escalation of therapy in a sequential manner is needed to maintain glycaemic control, progressing to combination treatment with a variety of therapeutic agents, including injectable and oral anti-diabetic (OAD) medications. Insulin is often ultimately needed to control hyperglycaemia.
The type of insulin and number of daily injections are essential considerations when choosing a particular regimen. Early and aggressive insulin use in the setting of progressive deterioration of metabolic control is advocated in patients with T2D to attain recommended glycaemic targets.2,3 Unfortunately, the initiation of insulin is commonly delayed in clinical practice until the disease is advanced and vascular complications have set in.4 Clinicians too often exhibit inertia and concerns about hypoglycaemia, weight gain, and increased cardiovascular risk. Such concerns have been shown to be largely unfounded.5,6
As with any other medication, a critical element is the proper use of insulin. The concept of basal-bolus therapy relies on daily multiple-dose insulin injections attempting to mimic healthy pancreatic insulin release as closely as possible. Studies have demonstrated the benefits of both basal and short-acting mealtime insulin.7 Once or twice daily long-acting insulin is added to OAD agents which usually leads to improved glycaemic control.8 Hypoglycaemia, especially nocturnal, is a concern. Daily glucose monitoring is necessary to detect hypoglycaemia and titrate insulin to achieve optimal glycated haemoglobin (HbA1c) levels. The issue of glycaemic variability, which may play a role in heightened endovascular inflammation and oxidative stress, persists.9 Perhaps the most problematic barrier to achieving good glycaemic control with basal insulin is that of meal-associated glucose elevations.10 The latter contribute to the overall burden of hyperglycaemia more significantly when HbA1c levels are lower, but still above the target, for most patients.11 Basal insulin replacement can lower fasting and pre-meal glucose, but its limitation is revealed in the form of persistent postprandial hyperglycaemia.12 Therefore, meal-associated glycaemic excursions also need to be addressed in T2D if a true basal-bolus concept of insulin treatment is to be implemented.13 Each component of this regimen comes from a different type of insulin with a predictable pharmacokinetic (PK) profile and specific pharmacodynamic (PD) characteristics. Doses are adjusted based on daily self-monitored glucose readings with the aim of matching insulin to glucose, minimising glycaemic variations, and maintaining the desired HbA1c levels.
INSULIN OPTIONS
Insulins are classified according to duration of action: rapid, short, intermediate, and long-acting types. A combination of intermediate or long- acting (for example, neutral protamine Hagedorn [NPH] or insulin glargine/detemir) and short or rapid-acting insulin (for example, regular human insulin [RHI], or insulin lispro, aspart, or glulisine) is frequently necessary to achieve good glycaemic control that is as close as possible to the physiological pattern of insulin secretion. However, regimen complexity, patient preference, and other practical factors may dictate the treatment choice. Often, simplicity and convenience must be balanced against a possibly better metabolic control with a multidose approach. For example, a regimen requiring only one or two injections of long-acting insulin may encourage adherence, but lead to suboptimal prandial control. On the other hand, a true basal-bolus regimen of four or more daily injections may enhance control but discourage compliance.
Using different types of insulin can work well for motivated patients who self-monitor their blood glucose regularly and are not averse to injecting many times daily or mixing varying doses of insulin. For the majority, however, this method of insulin administration may be too overwhelming, inconvenient, and intrusive for daily living. One solution is to use insulins that are already mixed in a fixed ratio of short-acting and basal components. These ‘premixed insulins’ offer an attractive option of delivering both rapid and longer-acting insulin in a single convenient injection, and theoretically address fasting, nocturnal, and prandial aspects of glucose management. Because the primary barrier to achieving good glycaemic control remains the underuse of insulin, premixed formulations can help a large subset of patients who stand to benefit from it.
BIPHASIC INSULIN ANALOGUE PREPARATIONS
Insulin analogues are a synthetic product of recombinant DNA technology with improved time-action profiles similar to physiological insulin release. Rapid-acting analogues (RAA), intended to be injected immediately prior to a meal, have a faster onset and peak action than RHI, thus better matching postprandial glucose elevations and minimised intra-individual variability. The biphasic insulin analogues (BIA) are composed of a single type of RAA that is modified to have dual-action PK profiles: a short-acting peak and a longer basal component. They are, therefore, ‘biphasic’ rather than the traditionally available human premixed insulins (HPI) composed of NPH and RHI.
Biphasic insulin aspart (BIAsp) 70/30 and insulin lispro 75/25 are the most commonly used BIA. Both insulins are also available in a 50:50 ratio: aspart as NovoLog Mix 50/50 (North America) or NovoMix 50 (Europe), and lispro as Humalog Mix50/50™ (North America) or Humalog Mix50™ (Europe). These dual-release formulations combine a soluble, rapid-acting component with a protaminated insulin analogue portion that has a prolonged duration of action. A biphasic preparation with 70% rapid-release aspart has also been marketed (NovoMix 70). Compared with HPI, the BIA have a faster onset of action (5–15 minutes) and earlier peak (1–2 hours) for the first component and a relatively steady second component lasting up to 16 hours.14,15 These characteristics lead to an improved PD effect, with favourable biochemical and physiological blood glucose-lowering actions in vivo (Figure 1).
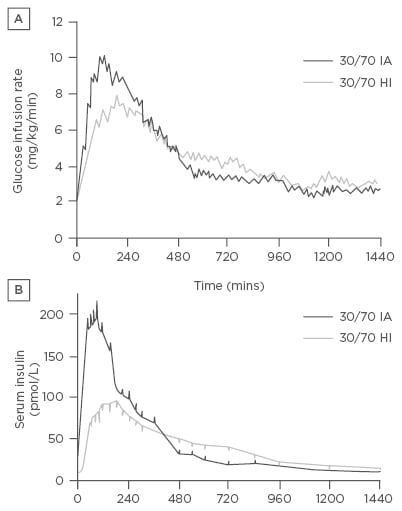
Figure 1: Pharmacokinetic profile of biphasic insulin aspart compared with human neutral protamine Hagedorn/regular human insulin.
A) Glucose infusion rates and B) serum insulin concentrations after a subcutaneous injection of either premixed rapid-acting analogue insulin aspart (30/70 IA) or a mixture of soluble RHI/NPH (30/70 HI).
IA: insulin aspart; HI: human insulin; NPH: neutral protamine Hagedorn; RHI: regular human insulin.
Copyright© 1997 American Diabetes Association. Reprinted with permission from The American Diabetes Association.15
How do BIA compare with HPI? The latter have long been a popular way to treat patients with diabetes. Their limitations include the inflexible necessity for injection 30 minutes before meals and a high incidence of hypoglycaemia, especially at night. BIA are conceptually similar, but because of their favourable PK and PD properties, they have a practical advantage.16,17 Patients can conveniently time their injections up to 15 minutes before eating, or even inject immediately after finishing a meal, if food patterns or amounts are unpredictable. This makes them more flexible and patient-friendly than HPI.18-20 Hermansen et al.21 demonstrated that both types of premixed analogues (lispro mix 25 and BIAsp 30) achieved better postprandial glucose control than HPI. Similar results were seen when Boehm et al.22 compared BIAsp 30 with HPI. BIA are associated with substantially less hypoglycaemia than HPI, especially nocturnally, which would conceivably translate into less treatment-related worry and higher acceptability for patients.23,24
THE CLINICAL EVIDENCE: HOW EFFICACIOUS ARE BIPHASIC INSULIN ANALOGUES?
The transition from non-insulin agents to insulin therapy in T2D can be achieved in several ways. A twice daily injection, before breakfast and the evening meal, is the most common method of using BIA and provides both basal and prandial coverage. Alternatively, another widely used treatment option for initiating insulin therapy is a once daily, basal-only injection of glargine. Luzio et al.25 showed that the PK and PD profiles were 28% and 32% higher and the residual endogenous insulin secretion, as measured by C-peptide concentration, was significantly suppressed after two subcutaneous doses of BIAsp 30 compared with one dose of insulin glargine. The authors suggested that an acute prandial delivery of insulin may protect the pancreas from excessive stimulation, thus providing β cell ‘rest’. In the 28-week, treat-to-target INITIATE study, BIAsp 30 was compared with insulin glargine.26 Both insulins were used in combination with metformin and initiated at the same dose. The HbA1c reduction was significantly higher with BIA in comparison to glargine. In the BIAsp 30 group, 42% reached an HbA1c of ≤6.5%, and 66% reached an HbA1c of <7.0%; the percentages in the insulin glargine group were 28% and 40%, respectively. Minor hypoglycaemia occurred more often in patients receiving premixed insulin than in those using insulin glargine (43% versus 16%, respectively).
The EuroMix study demonstrated that the biphasic insulins’ superiority over insulin glargine derived from their better control of postprandial glucose, since fasting glycaemia was similar with both treatment regimens.27 The risk of major hypoglycaemia was not increased in patients using BIA. Although the frequency of minor hypoglycaemic events was higher with premixed preparations, investigators and patients considered them acceptable and relatively easy to manage.
BIAsp 30 insulin can be used once daily as initial insulin treatment in patients with T2D. Garber et al.28 showed that 41% of patients receiving once daily BIAsp 30 reached an HbA1c level of <7.0% at the end of the first 16-week phase of the study, and the HbA1c level decreased from 8.6% to 6.6%. Treatment was intensified at 16-week intervals to aim for an HbA1c of <6.5%; if that goal was not reached, patients added a second and then a third injection of BIAsp 30 after each phase. At the end of the study, 60% of patients had achieved an HbA1c of <6.5% and 77% of <7%. No major nocturnal hypoglycaemia was seen, and the frequency of minor hypoglycaemic episodes was not related to the number of injections. Patient well-being seemed to be related to the level of control achieved.
BIAsp 30 plus metformin and thiazolidinedione showed superior control versus metformin plus thiazolidinedione alone, and 76% of patients reached an HbA1c target of <7%.29 Minor hypoglycaemia was acceptable, with approximately eight events per year. In insulin-naïve patients, BIAsp 30, administered twice daily, was similar in efficacy to a basal-bolus regimen of detemir and aspart; hypoglycaemia rates were low and similar between these groups.30
A landmark trial that addressed the merits of three different regimens for initiation of insulin treatment in T2D deserves mention. The 4-T Study Group enrolled 708 patients who were suboptimally controlled on metformin and sulphonylureas (HbA1c: >7%).31 At 1 year, mean glycated haemoglobin levels were similar in the biphasic and prandial groups (7.3% and 7.2%, respectively, p=0.08) but higher in the basal group (7.6%, p<0.001 for both comparisons). At 3 years, median HbA1c levels were similar for patients receiving biphasic, prandial, and basal insulin-based regimens (7.1%, 6.8%, and 6.9% respectively, p=0.28). Median rates of hypoglycaemic events per patient per year were lowest in the basal group (1.7), higher in the biphasic group (3.0), and highest in the prandial group (5.5) (p<0.001 for the overall comparison). The mean weight gain was higher in the prandial group than in either the biphasic group or the basal group. The investigators concluded that the addition of either a long or rapid-acting mealtime insulin to oral agents, followed by escalation to basal-prandial therapy, resulted in better glucose control and less hypoglycaemia than an initial biphasic insulin-based regimen, though the outcomes were not markedly different or statistically significant. Importantly, no matter how insulin treatment was started, achieving the target HbA1c level in most patients necessitated escalation of therapy to a full basal-bolus regimen, demonstrating the need for both fasting and prandial insulin coverage.
BIAsp 30 was initiated safely and effectively in insulin-naïve subjects with T2D in a largely primary care-based setting in Sweden.32 The mean HbA1c at baseline was 8.8% and improved to 7.2% after 6 months of treatment. In slight contrast, a retrospective database review of insulin initiation in 4,045 adults with T2D in UK primary care showed that few patients achieved glycaemic control targets with either basal or premixed and biphasic insulin regimens.33 The risk of severe hypoglycaemia when BIA are started in an outpatient setting is relatively low.34 Clinical and economic outcomes were similar in T2D patients who added RAA to glargine and in those who switched to HPI and BIA, although the former was associated with better treatment persistence and adherence.35
More recently, Riddle et al.36 demonstrated that a twice daily protamine-aspart/aspart insulin regimen was non-inferior to basal plus a single prandial dose, and a full basal-prandial approach was only slightly more efficacious. A biphasic insulin lispro 50/50 (Humalog Mix50) stepped-up treatment achieved glycaemic control similar to that with BIAsp 30.37 Malek et al.38 showed equivalent glucose control with thrice daily BIAsp 30 and a basal-bolus regimen of insulin detemir plus insulin aspart in insulin-naïve T2D patients. Similarly, a comparison of thrice daily insulin lispro premix with basal-bolus (insulin glargine once daily plus thrice daily prandial insulin lispro) therapy in approximately 400 Asian patients showed a similar degree of efficacy (HbA1c change at Week 24 being -1.1% for both treatment groups) and frequency of adverse events.39
The GALAPAGOS study was a 24-week, open-label, multinational trial that randomised uncontrolled insulin-naïve T2D patients on OAD to glargine and once or twice daily premixed insulin.40 Both regimens resulted in similar percentages of patients who achieved glycaemic goals without encountering severe hypoglycaemia. More patients achieved target HbA1c with premixes, whereas symptomatic hypoglycaemia was less with glargine.
Giugliano et al.41 conducted a systematic review and meta-analysis of randomised controlled trials looking at intensification of insulin therapy with basal-bolus or premixed (both HPI and BIA) insulin regimens in T2D. Thirteen studies lasting 16–60 weeks and involving 5,255 patients were included in the analysis. There was no statistically significant difference in hypoglycaemia event rate (0.16 episodes per patient per year), weight change, and daily insulin dose. The likelihood of attaining an HbA1c <7% was only 8% higher with the basal-bolus as compared with the premixed insulins. The authors concluded that there was no clinically relevant difference in the efficacy of the two regimens with regard to HbA1c lowering. Recent data on the use of biphasic insulin analogues in combination with dipeptidyl peptidase (DPP) 4 inhibitors has been published.42 In the 24-week Sit2Mix trial, Linjawi et al.42 randomised 582 insulin-naïve subjects on metformin to three groups. HbA1c reduction was superior with twice daily BIAsp 30 plus sitagliptin versus both once daily BIAsp 30 plus sitagliptin and twice daily BIAsp alone; the final HbA1c values were 6.9%, 7.2%, and 7.1%, respectively, from an average baseline of 8.4%. Hypoglycaemia and weight parameters favoured the once daily BIAsp 30 plus sitagliptin group.
Finally, a 24-week, randomised, open-label trial evaluated the efficacy and safety of biphasic 75/25 insulin lispro-protamine suspension twice daily versus basal insulin glargine plus once daily insulin lispro timed at the main meal in 476 patients; improvement in glycaemic control with insulin intensification was similar with both regimens.43 Table 1 summarises the data from several large clinical trials that studied biphasic analogue use in patients with T2D.
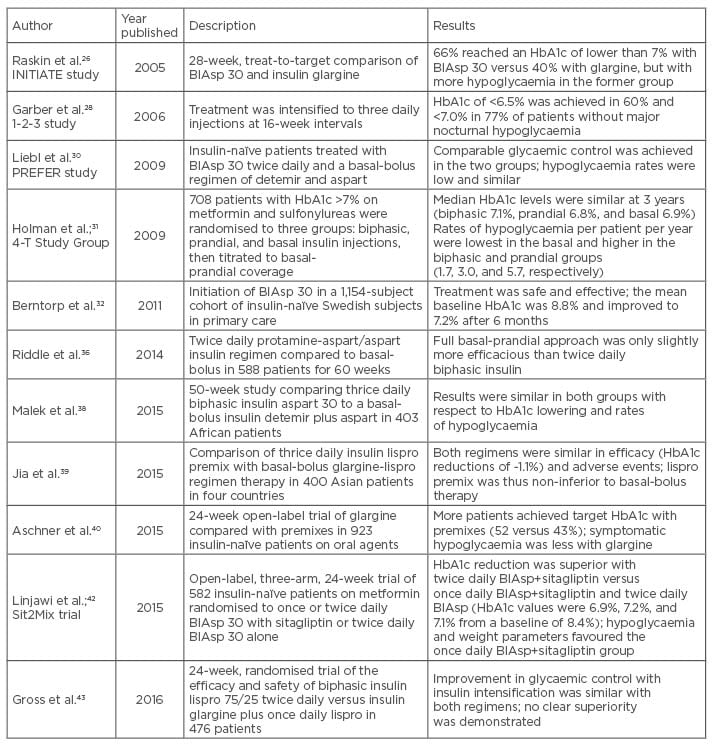
Table 1: Data from selected clinical trials involving biphasic insulin analogues in Type 2 diabetes.
BIAsp: biphasic insulin aspart; HbA1c: glycated haemoglobin.
PRACTICAL USE OF THE BIPHASIC ANALOGUES
Clinical evidence, summarised in Table 2, suggests that BIA are associated with desired improvements in overall and postprandial glycaemic control, low rates of hypoglycaemia, and good patient acceptance. In the realm of combination insulins, the theoretical advantage of BIA is that they provide a better replication of physiological insulin secretion than HPI. Providers have to consider several factors when deciding whether a patient is a suitable candidate for a biphasic insulin formulation. They may be considered as therapeutic options in the clinical situations discussed below.
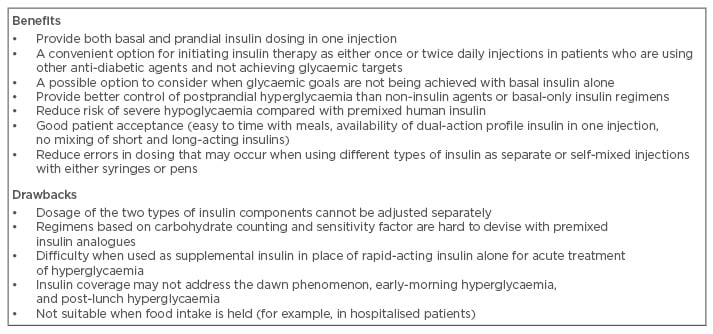
Table 2: Benefits and drawbacks of biphasic insulin analogues use in patients with Type 2 diabetes.
Initiation of Insulin Therapy
Patients with T2D and suboptimal glycaemic control on OAD therapy are candidates for insulin treatment. However, insulin may be introduced earlier if contraindications to other agents exist, or even at or soon after diagnosis if there is significant, symptomatic hyperglycaemia. A convenient single-injection combination of rapid and long-acting insulin, initiated as once or twice daily dosing, may provide an attractive and effective option compared with a basal-only or a complex multidose insulin regimen.44,45 One or two doses of long-acting injections daily, though popular, lack prandial coverage. The basal-bolus option requires more frequent injections of different types of insulin either separately or mixed by the patient in the same syringe. Finally, the inherent PK profiles of HPI do not match blood glucose as closely as those of BIA.
The ADA-EASD consensus statement contains recommendations on the continuing use and adjustment of existing anti-diabetic agents upon initiation of insulin therapy.1 Customisation to specific clinical situations and patient characteristics makes sense. If possible, metformin should be continued in insulin-treated patients. Combining insulin with a sulfonylurea may be of least clinical advantage, since it has the propensity to increase hypoglycaemia. The presence of comorbid conditions such as nephropathy and congestive heart failure may contraindicate the use of metformin and thiazolidinedione with insulin. The incretin-based therapies (glucagon-like peptide-1 analogues and dipeptyl peptidase inhibitors) may be combined with insulin. Careful assessment and individualisation is therefore essential for safe and effective use of BIA in combination with other therapies.
Targeting Postprandial Hyperglycaemia and Advancing to Basal-Bolus Therapy
Employing basal insulin such as NPH, insulin glargine, or insulin detemir alone or in combination with OADs is a popular approach in the treatment of patients with T2D. However, this strategy does not adequately address postprandial glucose elevations, and lowering of HbA1c levels to <8% remains difficult.13 Patients who are no longer achieving optimal control with basal insulin alone require progression to a basal-bolus regimen. At this juncture, adding RHI or a rapid-acting insulin before meals becomes necessary, leading to a significantly increased burden of daily injections for the patient.46 A more convenient option is to switch to twice daily BIA, thus providing basal and mealtime insulin coverage for most of the day without an unacceptable increase in the number of injections.27
Minimising Treatment-Related Hypoglycaemia
The occurrence of late postprandial and nocturnal hypoglycaemia is a risk with human insulins (e.g. RHI, NPH, or HPI). A better match of insulin to ambient blood glucose levels afforded by premixed analogues, and their lack of a significant peak effect, reduces insulin-glucose disparity and alleviates hypoglycaemia.22,23 Switching from premixed human to premixed analogue insulin may be helpful in this regard.
Convenience
Single-injection insulin combinations do not require mixing of two different products, and BIA can be given immediately before or after a meal. The enhanced flexibility and convenience improves quality of life and treatment satisfaction, and improves adherence to therapy.47,48 Availability in the form of disposable pens obviates the need to draw up insulin from a vial. After its first use, the insulin aspart 70/30 pen can be used for up to 14 days and the insulin lispro 75/25 and 50/50 pens for up to 10 days if kept at room temperature (below 86°F).49,50,51 These features are beneficial for patients with sight and dexterity issues, are more convenient for eating out and travel, and can reduce errors in dosing.52
Physician-Directed Self-Titration Regimen
Results of clinical studies provide guidelines for successful implementation and dose adjustment of premixed insulins in ambulatory practice.26,27 Suggested guidelines for dosing and titration, orientated to self-monitored blood glucose readings, are given in Table 3. The goal is to tailor the insulin regimen to the glycaemic profile of the individual patient.
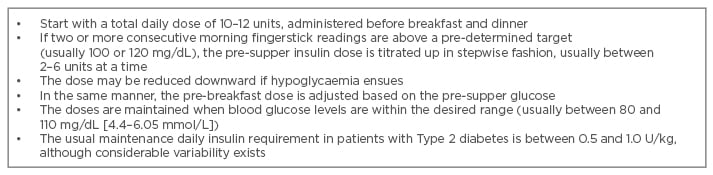
Table 3: Recommendations for the initiating and titrating of biphasic insulin analogues in clinical practice.
A limitation of this approach is that the rapid and long-acting components cannot be individually adjusted without modifying the total dose.53 As glycaemic control improves, the contribution of postprandial glucose to glycaemic burden increases,11 and the basal-bolus insulin requirement shifts according to the glycaemic pattern. BIA with a higher proportion of the rapid-acting component (lispro 50/50, BIAsp 50, and NovoMix 70) have been developed to overcome this problem. Similarly, glucose elevations associated with the midday meal may not be adequately covered with two injections and may require either three biphasic injections or a lunchtime dose of rapid-acting insulin. Patients who utilise carbohydrate counting and require varying amounts of mealtime insulin may find it difficult to use single-injection combination insulins to optimal advantage. As a caveat, the pre-dinner dose of an analogue may not last long enough, allowing early-morning hyperglycaemia to manifest; the so-called dawn phenomenon. Occasionally this issue has to be addressed by using separate doses of rapid and long-acting insulins at dinner and bedtime, respectively. Finally, patients treated with BIA may require an ‘as-needed’ supplemental dose of rapid-acting insulin for managing episodes of extreme hyperglycaemia during sickness or stress. Finally, when the rapid-acting insulin must be held prior to a diagnostic or surgical procedure, it may be preferable to administer a dose of long-acting rather than biphasic insulin.
CONCLUSION
The progressive nature of T2D is borne out by the fact that most patients ultimately require insulin to achieve optimal glucose control. With a wide variety of choices currently available, an insulin regimen individualised to each patient’s goals and preferences, rather than a universal approach, is recommended. Single-injection insulin analogues that contain two different formulations provide a basal-bolus therapeutic approach in a manner that is convenient and patient-friendly. If used judiciously, BIA can treat both fasting and postprandial hyperglycaemia while maintaining an acceptably low risk of hypoglycaemia. They remain a viable choice in clinicians’ armamentarium for helping patients with T2D achieve their goals.








