Abstract
The commensal bacteria that are present in our body since infancy are known to play a role in metabolism, in health as well as disease. Diabetes is a growing epidemic, and a long-term solution that targets the disease at the molecular level is yet to be developed. In this article, we have reviewed the link between the body’s microbiota and disturbed glucose metabolism, as well as the reasons for bacterial dysbiosis and the mechanisms by which it causes inflammation. The link between dysbiosis and diabetes is convincing, particularly since probiotics have been shown to be of some benefit in normalising disturbed metabolism in diabetes patients. Probiotics have recently been found to have a wide application in diseases such as autoimmune, inflammatory, and allergic conditions. The efficacy of probiotics in diabetes has been proven by their ability to lower fasting glucose and insulin levels in a preclinical setting as well as in human trials. However, there is heterogeneity in these studies, including the species used, probiotic dosage, and the magnitude of efficacy. Based on the robust understanding of the benefits of probiotics in diabetes at the cellular level, in both animal studies and clinical trials, combined with their excellent tolerability, probiotics should be explored for their application in clinics.
INTRODUCTION
Bacteria enter the body at the time of birth, growing in diversity and numbers to resemble the adult composition by around 2–3 years of age.1 They are not just commensals but are mutualistic, since both humans and the bacteria benefit from each other. The number of bacteria equals the number of human cells, at a ratio of 1:1 (previously thought to be 10:1).2 From the perspective of this vast number, the flora can be referred to as a separate endocrine organ. This flora, which we gradually build from birth, influences the body’s functions. Nearly all organs that come into contact with the external environment harbour commensal bacteria, such as the gastrointestinal tract, skin, saliva, oral mucosa, and conjunctiva, with the colon harbouring the largest number of bacteria.2 Some of the well-known physiological roles of these bacteria are the production of vitamins in the gut,3 maintaining an acidic pH in the vagina,4 and preventing outgrowth of pathogenic bacteria. Metabolomic studies have shown the significant influence of these bacteria on important biochemical reactions and metabolic pathways. Research has also suggested the role of the microbiome in a vast array of diseases, such as diarrhoea,5 bacterial vaginosis,4 and allergic6 and autoimmune inflammatory conditions, including Crohn’s disease, ulcerative colitis, and irritable bowel disease.7 The gut has also been linked via these bacteria to the central nervous system in conditions such as depression, stress, and anxiety.8
Metabolic syndrome and diabetes are conditions that now deserve to be recognised as being of high importance. The widespread use of antibiotics, improved sanitation, and the transition to a highly processed diet lacking fibre and other prebiotics, have altered our natural flora.9 There are several observations that have led us to believe that this altered flora, or dysbiosis, is linked to metabolic diseases such as diabetes, obesity, hypertension, and dyslipidaemia.
The objective of this review was to first understand the reason behind an altered flora in these patients, followed by a review of how dysbiosis affects carbohydrate metabolism. In the second part of this article, the beneficial role of probiotics in modulating glycaemic parameters has been explored, at the in vitro level as well as in animal and human studies.
Evidence was searched for using PubMed and Google Scholar, by entering key words such as ‘altered flora’, ‘dysbiosis’, ‘obesity’, ‘diabetes’, and ‘probiotics’. Original research articles detailing animal and human studies, review articles, and meta-analyses were reviewed, and finally all relevant data were compiled.
UNDERSTANDING THE LINK BETWEEN ALTERED GUT FLORA AND DIABETES
Altered Gut Flora in Obese People with Diabetes
Data from animal and human models suggest that obesity and Type 2 diabetes mellitus (T2DM) are associated with a profound dysbiosis.10 Gut bacteria is an important determinant of susceptibility to obesity and related metabolic diseases. Gut bacteria could also affect obesity by promoting a chronic inflammatory status.11
Animal models of obesity have made connections between an altered microbiota composition and the development of obesity, insulin resistance, and diabetes in the host through several mechanisms, such as increased energy harvest from the diet, altered fatty acid metabolism and composition in adipose tissue and the liver, modulation of gut peptide YY and glucagon-like peptide 1 secretion, activation of the lipopolysaccharide toll-like receptor-4 axis, and modulation of intestinal barrier integrity by glucagon-like peptide 2.12
Differences Between Gut Flora in People with and Without Diabetes
The relationship between the gut microbiota and human health is becoming increasingly recognised. It is now well-established that a healthy gut flora is largely responsible for overall health of the host. The normal gut microbiota has specific functions in host nutrient metabolism, xenobiotic and drug metabolism, maintenance of structural integrity of the gut mucosal barrier, immunomodulation, and protection against pathogens.13
It is reported that the gut microbiota between adults with T2DM and nondiabetic adults is quite different.14 In landmark research from a European Union (EU) supported research team with European and Chinese researchers, MetaHIT clearly showed that specific imbalances in the composition and function of the intestinal bacteria led to insulin resistance and thereby increased the risk of developing T2DM.15 Gut microbial dysbiosis and an increase in opportunistic pathogens, along with a reduction of butyrate producing bacteria, were seen in patients with T2DM.16
Connections Between Gut Leakage, Inflammation, and Glucose and Insulin Metabolism
Whole bacteria, as well as their products and metabolites, undergo increased translocation through the gut epithelium to the circulation, due to degraded tight junctions and the consequent increase in intestinal permeability that culminates in inflammation and insulin resistance.17 Lipopolysaccharides (LPS) from the membranes of gram-negative bacteria penetrate into the blood stream via impaired permeability of the intestinal mucosa, which induces metabolic endotoxemia, inflammation, impaired glucose metabolism, insulin resistance, obesity, and contributes to the development of metabolic syndrome, T2DM, and other conditions.18
Dietary intolerance (excessive fat, refined carbohydrate, or fructose) alters the gastrointestinal microbiota. This initiates an immune response when bacteria migrate into the general circulation, as a result of increased intestinal permeability. Increased insulin resistance in the hypothalamus increases food intake by decreasing satiety, thus increasing body weight. Consequential inflammation and immune cell infiltration of insulin sensitive organs induces insulin resistance, supporting the obese phenotype.19
Dysbiosis and the resulting increased LPS levels induce chronic inflammation.20 The mechanism involved alters the commensal bacteria proportions in the gut and reduces the expression of adhesion proteins and tight junction proteins in the intestinal mucosa, which leads to increased permeability and translocation of LPS.21 Type 1 diabetes mellitus (T1DM) patients were found to have an altered thickness of microvilli and the space between microvilli, as well as a reduced thickness of tight junctions.22 In addition to LPS, bacterial fragments also diffuse through the intestinal barrier and bind to pattern recognition and toll-like receptors that regulate innate and adaptive immunity.23 In a separate study, it was observed that in mice with high-fat diet-induced diabetes, live bacteria, originating in the intestine, translocated to the blood and adipose tissue where low-grade inflammation was initiated.24 In another study, it was revealed that metabolic endotoxaemia due to bacterial LPS caused hyperglycaemia, hyperinsulinaemia, and insulin resistance, and this action of LPS was mediated through CD-14 receptors.25
A study of women with T2DM identified that these patients had a different gut metagenome compared to those with normal glucose control; this implied that the metagenome can be used as a risk factor to predicting T2DM.14 Apelin, a molecule with the potential to restore insulin sensitivity, can induce glucose lowering by enhancing glucose uptake into the skeletal muscles and adipose tissue (Figure 1).26
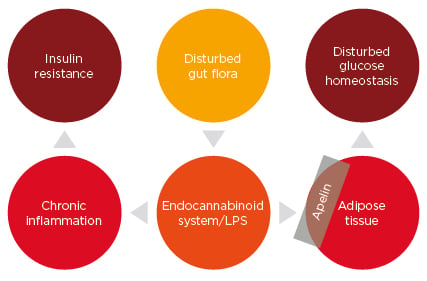
Figure 1: A possible mechanism of regulation of glucose homeostasis by gut bacteria.
LPS: lipopolysaccharide.
Conditions Predisposing to Altered Gut Flora
High dietary fat, obesity, and diabetes all predispose individuals to an altered microbiota. It was found that in rats given a high-fat diet, the proportions of Bacteroides, Clostridium, and Enterobacteriaceae species increased, whereas the total bacterial density was reduced.27 Gut microbial dysbiosis and an increase in opportunistic pathogens, along with a reduction of butyrate producing bacteria, were seen in patients with T2DM.16 One of the reasons for an altered gut flora associated with a high-fat diet can be an altered bacterial adhesion to the gut mucosa.28 In another discovery, the fasting and fed states in mice correlated with a reduction and an increase in metabolic endotoxaemia, respectively. Furthermore, when the mice were given a high- fat diet for 4 weeks, the levels of LPS-containing bacteria in the gut increased chronically.25
The Gut-Adipose-Glucose Axis
Dysbiosis can increase the endocannabinoid, anandamide, levels in the adipose tissue, as studied in an obese diabetic mouse model. Also, dysbiosis leads to increased LPS levels, which induce low-grade inflammation in the gut.29 These two factors, endocannabinoid tone and LPS, regulate the expression of apelin, which is secreted by the adipose tissue.30
Chronic Inflammation Due to Altered Bacterial Flora and Diabetes
Chronic inflammation has been recognised as an important factor in the pathogenesis of diabetes and obesity. This finding is supported by a vast amount of research. Inflammatory cytokines cause resistance to leptin and insulin. The mechanisms that link inflammation with insulin resistance include the activation of IκB kinase complex (inhibitor of kappa beta, a kinase which activates inflammation), extracellular signal-regulated protein kinases 1 and 2 (ERK1/2), and c-Jun N-terminal kinases. These changes cause insulin resistance by decreasing tyrosine phosphorylation of the insulin receptor substrate proteins.23 The expression of genes encoding insulin receptor substrate-1, GLUT4, and PPAR-α are affected by cytokines produced in the adipose tissue, such as tumour necrosis factor (TNF)-α or interleukin (IL)-1β.31
Preclinical Evidence Supporting the Role of Probiotics in Diabetes
As defined by the World Health Organization (WHO), probiotics are live microorganisms, which, when administered in adequate amounts, confer a health benefit on the host. Immense research has been conducted on probiotics in recent decades, with several bacterial and some fungal organisms being used as probiotics. As well as varied applications in human health, probiotics hold a strong place in veterinary science, being used as feed supplements. Strains like Enterococcus faecium can cause opportunistic diseases in humans and are only used as probiotics in animals. The majority of the probiotic strains belong to the lactic acid bacteria that can convert sugars to lactic acid, thus inhibiting pathogen growth by creating an acidic environment. Some commonly used probiotic strains with health benefits have been compiled in Table 1.32 The role of probiotics in glucose homeostasis and diabetes is reviewed hereafter.

Table 1: A list of commonly used probiotic organisms.
Adapted from Fijan.32
Probiotics Improve Epithelial Barrier Function
In an epithelial cell model, Lactobacilli were found to reinforce the barrier function of epithelial cells by increasing the levels of adhesion proteins, including beta-catenin and E-cadherin. They also stabilised the junctional E-cadherin/ beta-catenin complex by stimulating a protein kinase.33
Probiotics Reduce Pathogen Adherence in the Intestine and Translocation to Adipose Tissue
In a model of high-fat diet-induced diabetes in mice, after 4 weeks of a high-fat diet, the counts of Escherichia coli increased in the intestine. As the strain was radiolabelled, tracing of the same bacterial species also showed translocation from the intestine to the blood and mesenteric adipose tissue. One month of daily probiotic treatment with Bifidobacterium animalis reduced the counts of Enterobacteriaceae species present in both the small intestine mucosa and the adipose tissue.24 In another study, it was found that 12 different probiotic strains were all effective in displacing Bacteroides, Clostridium, Staphylococcus, and Enterobacteriaceae from immobilised human mucus.34
Probiotics Modulate Immune Differentiation
Lactobacillus johnsonii was found to induce T helper (Th)17 cell differentiation in mesenteric lymph nodes, and thereby provided immunity to the development of T1DM in diabetes prone rats. Another group treated with Lactobacillus reuteri did not develop Th17 differentiation and, thereafter, developed T1DM.35 One month of daily probiotic treatment with B. animalis was found to reduce the expression of proinflammatory cytokines, such as TNF-α, IL-1β, plasminogen activator inhibitor-1, and IL-6, in the mesenteric adipose tissue, liver, and muscle (as determined by the concentration of coding messenger RNA).24 However, in a clinical study of patients with T2DM, probiotic supplementation failed to reduce markers of inflammation when given for 4 weeks.36
Probiotics Normalise Insulin Sensitivity
Probiotic administration of Bifidobacterium for 1 month in mice with high-fat diet-induced diabetes normalised the glucose metabolism. This was documented by the lowering of fasting insulin levels, blunting of glucose excursions on the intraperitoneal glucose tolerance test, and increase in the glucose turnover rate.24 In a genetic mouse model of T2DM, administration of the Lactobacillus gasseri probiotic twice a day orally for 12 weeks resulted in a significant fall in fasting and post-prandial glucose levels.37
Clinical Evidence Supporting the Role of Probiotics in Diabetes
Effect on glycaemic parameters
Clinical trials of probiotic use in patients with diabetes, as well as healthy individuals, are summarised in Table 2. Probiotics were found to lower fasting blood glucose, insulin levels, and improve glycosylated haemoglobin (HbA1c) and insulin resistance (as measured by lowering of HOMA-IR [Homeostatic model assessment for insulin resistance] values).
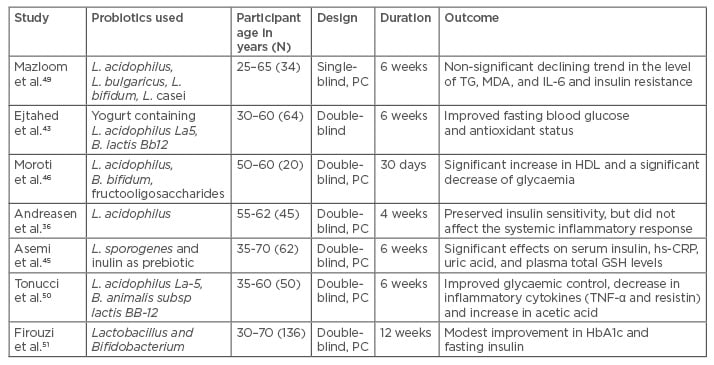
Table 2: Overview of important clinical studies demonstrating the effect of probiotics on metabolic profiles in patients with Type 2 diabetes mellitus.
B. species: Bifidobacterium; GSH: glutathione; HbA1c: glycated haemoglobin; HDL: high-density lipoprotein; hs-CRP: high-sensitivity C-reactive protein; IL: interleukin; L. species: Lactobacillus; MDA: malondialdehyde; PC: placebo controlled; TG: triglyceride; TNF: tumour necrosis factor.
A meta-analysis of 12 randomised controlled trials (RCT) with reported lipid profiles (n=508), fasting blood glucose (n=520), HOMA-IR (n=368), and HbA1c (n=380) concluded that probiotics could reduce fasting blood glucose levels by around 15 mg/dL (0.8325 mmol/L) and HbA1c by 0.54% (3.6000 mmol/mol), along with a significant improvement of 0.98 in HOMA-IR values, indicating a modest effect on glycaemic control. This analysis elucidated that probiotics may improve glycaemic control and lipid metabolism in T2DM.38
Another meta-analysis of 11 RCT with 614 subjects concluded that the glucose reduction was 9.36 mg/dL (0.52 mmol/L; 95% confidence interval [CI]: -0.92–[-0.11] mmol/L; p=0·01) and the HbA1c reduction was 0.32% (1.1 mmol/L; 95% CI: -0·57–[-0·07%]; p=0·01). The reduction in insulin levels and improvement in HOMA-IR was significant in patients with diabetes. This suggested that probiotics may be used as an important dietary supplement in reducing the glucose-related metabolic factors associated with diabetes.39 Overall, there was a modest effect on glycaemic control in both meta-analyses in patients with diabetes.
A recent meta-analysis of 12 RCT involving 770 patients with T2DM showed that probiotics could significantly reduce fasting blood glucose by 11.27 mg/dL (95% CI: -21.76–[-0.79]; p<0.001) and serum insulin concentration by 2.36 μU/mL (95% CI: -4.01–[-0.72]; p=0.005), but with no significant reduction in HbA1c (-0.19%; 95% CI: -0.49–0.12; p=0.230). Probiotics could significantly reduce HOMA-IR in T2DM patients (-1.05; 95% CI: -1.52–[-0.59]; p<0.001).40
Non-alcoholic fatty liver disease (NAFLD) is thought to be a hepatic manifestation of metabolic syndrome and could share its pathology with diabetes. A meta-analysis evaluated the effect of probiotics in patients with NAFLD. It reported that there was a significant reduction in the HOMA-IR values by 0.46 (95% CI: -0.73–[-0.19]; p=0.0008).41 A HOMA-IR value >2 is generally present in patients with metabolic syndrome.42
Effects on oxidative stress and inflammatory parameters
Probiotics improve antioxidant status in patients of T2DM. In a study, improvement of the antioxidant enzyme levels, such as glutathione peroxidase and erythrocyte superoxide dismutase, were noted.43 In a study of 72 patients with T2DM and NAFLD, probiotics given for 30 days were found to significantly reduce the levels of IL-6, IL-8, IL-1, interferon-gamma, and TNF-α.44 Synbiotics (probiotics and prebiotics) were found to significantly reduce the levels of high-sensitivity C-reactive protein in a clinical study on T2DM patients.45
Effects on lipid parameters
It has been documented that probiotics increase high-density lipoprotein.46 The widely reported mechanism suggests that cholesterol is lowered due to the prevention of bile salt recycling, since probiotics deconjugate the bile salts, which are then not able to be reabsorbed and are excreted.47
Tolerability
In the clinical trials previously discussed, no adverse events were reported with probiotics in patients with T2DM. Probiotics were found to be safe to use in healthy pregnant women, who did not report any adverse effects. No adverse events were seen in their children either.48 Use of probiotics in immunocompromised individuals is known to cause concern.
DISCUSSION AND CONCLUSION
Probiotics have been proven to be effective in diseases such as infective diarrhoea, chronic inflammatory bowel diseases, lactose intolerance, and allergy, and we have reviewed their role in diabetes. The link between pathogenic bacteria and chronic low-grade inflammation is established. The mechanisms by which inflammation leads to insulin resistance are also well-defined. Intervention to restore normality of the gut flora by means of probiotics has led to improvement in glycaemic parameters, as shown in clinical trials. Lactobacillus and Bifidobacterium species have been the most commonly used probiotics in these trials. Since all bacteria have a different mode of action, the use of multiple species is preferable until trials localise a specific strain. The efficacy was improved when probiotics were used for a moderate to long duration (around 8 weeks). However, conflicting results were revealed in some trials where probiotics did not have any effect on glycaemic parameters. This could be because of sub- therapeutic doses or insufficient study duration.
Although evidence is limited, the use of probiotics as add-on agents is advocated, keeping in mind the strong preclinical evidence as well as clinical evidence in improving diabetes, along with their excellent tolerability profile. Further research needs to be performed on selecting the exact strain and therapeutic dose, as well as study duration, for optimum effect.








