Abstract
The management of adults with Type 2 diabetes mellitus (T2DM) was traditionally delivered in a single specialist setting with a focus on glycaemic control. As the treatment landscape evolved to consider the need to prevent cardiovascular disease and/or microvascular complications, so did the requirement to manage this complex multisystem condition by multiple healthcare providers in both primary care and specialist settings. This article discusses the key studies that changed the way T2DM is managed to incorporate an interdisciplinary approach to care, the principles of the multidisciplinary teams, examples of multidisciplinary teams in real-world clinical practice, and associated patient outcomes.
INTRODUCTION
Diabetes is a global epidemic affecting an estimated 425 million adults aged 20–79 years. In 2017, there were 58 million individuals in Europe with diabetes and this figure is set to rise to 67 million by 2045.1 Adults with Type 2 diabetes mellitus (T2DM) make up 90% of all patients with diabetes.2,3 T2DM prevalence is increasing due to population ageing, changes in dietary behaviours, obesity, and sedentary lifestyles, all of which have severe implications for healthcare systems in terms of the morbidity and cost burden.3,4 There is a large unmet need to streamline services using multidisciplinary teams (MDT) for optimal management of the large number of patients with T2DM.
T2DM pathogenesis is multifactorial and characterised by a combination of increased glucose production, impaired insulin secretion by pancreatic beta cells, and the development of peripheral insulin resistance. For T2DM to occur, both insulin resistance and inadequate insulin secretion must exist.5,6 T2DM morbidity and the correlation between hyperglycaemia and vascular complications results from multiple biochemical pathways. Individuals with T2DM may experience cardiovascular disease (CVD) and/or microvascular complications that affect the kidney, retina, and nervous system.3,5,7-9 Complications in patients with T2DM are common, with approximately 27% and 50% of patients experiencing macrovascular and microvascular complications, respectively.3
DIABETES TREATMENT LANDSCAPE PROGRESSION AND EVOLUTION TOWARDS A MULTIDISCIPLINARY APPROACH
The T2DM treatment landscape has evolved considerably over the past 40 years. The clinical endpoints that physicians use to determine the optimal care of patients has changed from glycaemic control (HbA1c) to a focus on prevention of macrovascular disease, in particular the prevention of cerebrovascular, renal, and cardiac disease.10 During this time, new agents and drug classes have become available that are effective in the prevention of these morbidities.11,12
DIABETES LANDSCAPE EVOLUTION CAN BE CLASSIFIED INTO SEVERAL TIME PERIODS:
- Before 1998 where control of glycaemia was assumed to be beneficial.
- 1998–2015 where glucose-lowering studies largely demonstrated reduction in microvascular events but raised concerns about CVD risk.
- 2015 onwards where studies of new glucose lowering therapies demonstrated cardiovascular (CV) and renal benefits in addition to improving hyperglycaemia.
The pre-1998 control of glycaemia-only approach was challenged by the UK Prospective Diabetes Study (UKPDS).13-15 The study commenced in 1977 and evaluated if long-term intensive blood glucose control by either sulphonylureas, insulin, or conventional treatment could reduce the risk of microvascular and macrovascular complications in 5,102 patients with newly diagnosed T2DM. Over a 10-year period, the UKPDS found that reducing glucose exposure from HbA1c 7.9% to 7.0% with sulphonylurea or insulin therapy, reduced the risk of ‘any diabetes-related endpoint’ by 12% and microvascular disease by 25%. A nonsignificant relative risk reduction for myocardial infarction (MI) of 16% (p=0.052) was also found.13,15 The legacy of UKPDS was that the achievement of tight glycaemic control could result in lower rates of microvascular complications but perhaps not CVD.13-15
As the UKPDS associated an HbA1c of 7% with better outcomes, further studies were conducted to determine if tighter glycaemic control to HbA1c 6.0–6.5% in patients with established T2DM was associated with additional morbidity benefits.14 Studies such as the Action to Control Cardiovascular Risk in Diabetes (ACCORD), the Veterans Affairs Diabetes Trial (VADT), and Action in Diabetes and Vascular Disease Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) study found that it was possible to achieve tighter levels of glycaemic control using conventional agents such as metformin, sulphonylureas, thiazolidinediones, and insulin, yet none demonstrated significant improvements in combined vascular end points.14,16-19 Furthermore, the ACCORD and VADT studies found that intensive management of glycaemia compared with standard approaches was associated with 20% increased mortality and a higher number of deaths (hazard ratio [HR]: 1.07; p=NS), respectively.16,18 Further concerns regarding the CV safety of agents used to manage patients with diabetes then emerged. In 2007, a meta-analysis evaluating rosiglitazone studies reported a significant increase in the risk of MI (odds ratio [OR]: 1.43; 95% confidence interval [CI]: 1.03–1.98; p=0.03), and an increased risk of death from CV causes (OR: 1.64; 95% CI: 0.98–2.74; p=0.06).20 These findings were of concern to physicians and they also changed the way new diabetes therapies were assessed as the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) issued a requirement that all new therapies for diabetes undergo assessment of CV safety through large-scale cardiovascular outcome trials (CVOT).21,22
This treatment landscape evolution was a new opportunity for the diabetologist and cardiologist, in the setting of a multidisciplinary approach, to concomitantly improve glycaemic control and reduce the risk of CV events in patients with T2DM. The benefits of multifactorial care involving intensive therapy with tight glucose regulation and administration of renin-angiotensin system blocker, aspirin, and lipid-lowering agents in patients with T2DM were beginning to be recognised. These included a lower risk of death from CV causes (HR: 0.43; 95% CI: 0.19–0.94; p=0.04) and of CV events (HR: 0.41; 95% CI: 0.25–0.67; p<0.001).23 The management of patients with T2DM progressed to a combined approach and in 2007, as part of ten practical steps for healthcare providers (HCP) to enable them to achieve their glycaemic goals, the Global Partnership for Effective Diabetes Management recommended the implementation of MDT.24
MULTIDISCIPLINARY TEAM APPROACH IN THE ERA OF CARDIOVASCULAR OUTCOMES TRIALS
Multiple trials have been performed that incorporate CV safety when evaluating the newer antihyperglycaemic drugs, such as sodium glucose co-transporter 2 (SGLT2) inhibitors and glucagon-like peptide 1 (GLP-1) receptor agonists.25 The first of the modern CVOT trials to show superiority over placebo was the Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes (EMPA-REG) study; this reported not only CV safety, but also a 38% reduction in CV death, a 35% reduction in hospitalisation for heart failure, and a 32% reduction in the risk of death from any cause.26 Other trials such as the CANagliflozin cardiovascular Assessment Study (CANVAS), in patients with T2DM and high CV risk treated with canagliflozin, demonstrated significantly lower risk of the composite outcome of major adverse CV events (MACE; CV death, nonfatal MI, or nonfatal stroke; HR: 0.86; 95% CI: 0.75–0.97; p<0.001), hospitalisation for heart failure, and improved renal outcomes. Further trials assessing these and other SGLT2 inhibitors have also shown CV and renal benefits, including a reduction in the risk of end-stage renal disease or renal death.27-33 Studies assessing GLP-1 receptor agonists, liraglutide, albiglutide, dulaglutide, and semaglutide have found significant reductions in composite major cardiovascular events (CV death, non-fatal MI, or stroke), and/or albuminuria.34-37
A positive outcome from CVOT in terms of the MDT approach was that they included assessments of CV safety with strict glucose control and the incorporation of the CVD standard of care. This was an important step in the management of patients with T2DM and an improvement from earlier trials that were undertaken before blood pressure-reducing drugs, statins, anti-platelet medications, and an active approach to coronary revascularisation were part of routine care for patients with T2DM.38 The high rates of chronic kidney disease (CKD) in patients with T2DM and the renal benefits associated with newer glucose-lowering therapies mean that nephrologists, in addition to cardiologists and endocrinologists, were increasingly included as part of the MDT.
MULTIDISCIPLINARY TEAM STRUCTURE, PRINCIPLES, AND CONCEPTS
The MDT approach should be focussed on integrated management with multiple treatment goals including glucose, blood pressure and lipid control, life style management, regular appointments, and screening for the prevention of T2DM morbidities.39,40 For those patients who are considered to have less complex clinical needs, integrated care with MDT should be anchored in the primary care setting.41,42 This structure has led to cost savings and a reduction of disease burden for healthcare systems related to fewer hospitalisations and vascular events.43 Whilst primary care physicians (PCP) are the first point of contact and a source of continuous comprehensive care, they do not work in isolation but involve other specialities, such as podiatrists, nurses, and dietitians.39
Patients with complex needs and high rates of morbidities are referred to endocrinologists and are typically seen in hospital outpatient settings.41 Optimal diabetes interdisciplinary care of these patients is complex and the number of HCP involved rises due to the need to prevent and manage multi-morbidities such as CKD and heart failure. The hospital-based team may include ophthalmologists, cardiologists, nephrologists, a diabetic foot team, and the PCP.39,40,44,45
The principles, key concepts, and core components for multidisciplinary care are displayed in Figure 1. All the MDT team members need to be actively involved to ensure an effective approach to the provision of care. Key concepts and principles include the importance of a team approach with shared responsibility and decision making, in addition to a respect for all team members and the MDT should also be mindful to the needs of the patient.42,46 The MDT approach must feature a continuity of care with well-defined processes and protocols that include appropriate referral pathways.
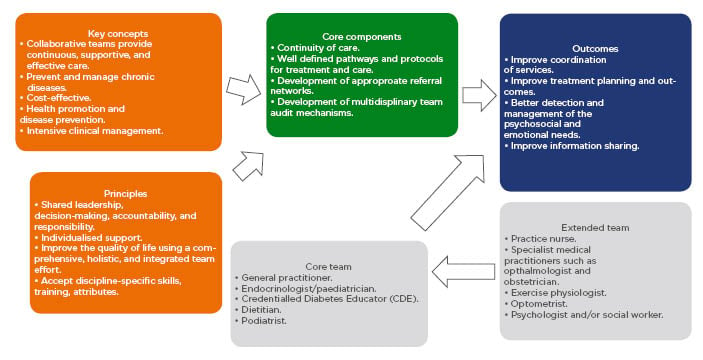
Figure 1: Key concepts, principles, members and pathways of typical multidisciplinary teams involved in the care of patients with Type 2 diabetes mellitus.
Adapted from The National Institute of Diabetes and Digestive and Kidney Diseases Health Information Center (NIDDK)42 and Australian Diabetes Educators Association (ADEA).46
Further to the MDT, optimal diabetes management programmes also include different components such as registration systems,39 audit and feedback, clinician reminders, patient and HCP education, and IT systems.
THE ROLE OF THE PATIENT IN MULTIDISCIPLINARY TEAMS
The role of the patient in the MDT must not be overlooked. Studies have shown that patients who do not participate in the MDT care approach are less likely to reach their treatment targets. A considerable proportion of diabetes management is undertaken by the patient, such as lifestyle modifications and treatment adherence. HCP have limited ability to control how patients manage their disease outside of visits. It is important that the MDT must consider the numerous variables that are outside their control but impact disease management and educate the patient accordingly to empower them to take an active role in their care. An investigation assessing patient (N=53) perspectives of MDT care reported barriers such as lack of co-ordination among many HCP and the large number of appointments they needed to attend with many different HCP.47 Yet, patients were strongly in favour of the team-based approach and stated that highly interdisciplinary teams (IDT) were desirable. Patients did not believe that diverse teams would be associated with fragmentation but appreciated having a single point of contact for their care. In conclusion, patients felt that appropriate management of T2DM was too complex for a single HCP, but co-located teams were more convenient.47
EXAMPLE OF NHS ENGLAND REAL-WORLD EXPERIENCE OF MULTIDISCIPLINARY TEAMS IN PRACTICE
In 2009, Portsmouth Hospitals NHS Trust identified that a lack of co-ordinated and communicated plans across HCP was one of the main barriers to improving the care of patients with T2DM. They developed a new model of care that transitioned most patients who were considered less complex out of specialist care. However, some patients still required care under the auspices of a specialist setting.43,48 Patients within one of the following six categories in the ‘Super Six Model’ remained within specialist care:41,43,48
- Patients on insulin pumps.
- Women with antenatal diabetes.
- Those requiring diabetic foot care.
- Patients with low estimated glomerular filtration rate (eGFR) or who require dialysis.
- Inpatients with T2DM.
- Patients with Type 1 diabetes mellitus (individuals with poor control or young people).
Yet, collaboration between PCP and specialists still occurred and these HCP maintain regular communication in addition to 6 or 12-monthly specialist consultations.43,48
This model was further expanded in Leicester, UK, whereby clinics were segregated according to different tiers and included patient education activities.48 The new system provided integrated care with supplementary services. Different tiers enabled PCP to manage increasingly complex patients and was proven to be cost-effective by reducing the healthcare resource burden associated with hospitalisation.48 Further similar initiatives have been implemented in Derby, Wolverhampton, and north-west London. Outlines of these models, pathways, and enablers are shown in Figure 2. All of the models rely on enablers that include:48
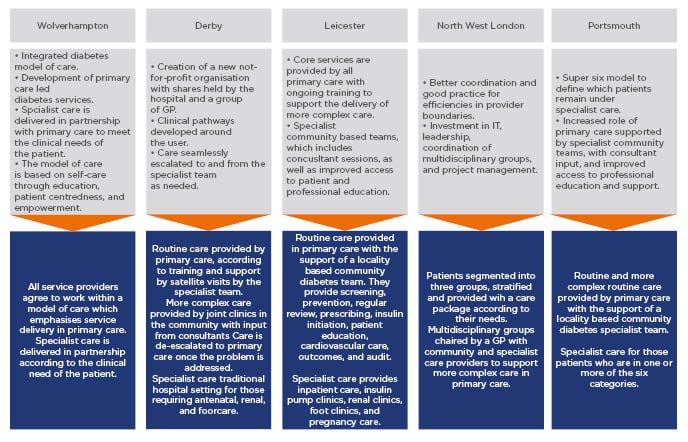
Figure 2: Examples of local initiatives to deliver models of integrated diabetes care in several UK locations.
GP: general practitioner.
Adapted from Diabetes UK.48
- A single central IT system used by both primary care providers and the specialist teams to enable rapid communication, accurate recording keeping, information dissemination, and appropriate referrals.
- Aligned finances and responsibilities which may include single budgets or trusts to remove boundaries, incentivised payments for primary care staff training.
- Engagement, networks, and leadership with MDT groups for particular workstreams or regular meetings to provide opportunities to discuss and identify efficiencies in the collaboration.
- Clinical governance, including integrated management boards, operational groups, monthly review boards with accountability and responsibilities to drive success, review outcomes, refine pathways, and ensure high quality service delivery.
OUTCOMES AND MULTIDISCIPLINARY TEAMS
MDT must be associated with improved outcomes for patients. Assessment of feasibility and effectiveness of IDT specifically has been assessed in a Belgian study that determined if the implementation of an IDT was feasible in a healthcare setting with historically low rates of shared care, and if patients who made use of an IDT would have improved outcomes over an 18-month period.49 A two-arm cluster randomised trial found that the use of the IDT was significantly associated with improvements in HbA1c (p=0.00001) and LDL-cholesterol (p=0.00039), an increase in the use of statins (OR: 1.902; p=0.04308), and anti-platelet therapy (p=0.00544).49 IDT also significantly increased the number of clinical targets reached (p=0.005). The results of this trial demonstrated that the use of IDT teams in primary care that are actively guided and supported by a specialist team are associated with important improvements in clinical outcomes.
A European-wide systematic literature review and meta-analysis of randomised controlled trials evaluated the effectiveness chronic care programmes for T2DM from January 2000 to July 2015.50 These programmes were characterised by integrative care and a multicomponent frame work for enhancing healthcare delivery compared with usual diabetes care. Of the seven trials, four evaluated the impact of MDT in addition to other factors such as the impact of guideline-based care, patient education, shared decision making, and annual screening in patients with either prevalent diabetes or screen-detected diabetes.50 Two of the trials reported no significant differences in HbA1c levels between intervention groups and control groups after 1 year. One study that assessed combined interventions from Denmark, the Netherlands, Cambridge, and Leicester over a 5-year period found significant improvements in HbA1c in the intervention group versus the control group (-0.08%; 95% CI: -0.14 to -0.02 versus -0.9 mmol/mol; 95% CI: -1.5 to -0.2). Of all the trials that assessed MDT, only the pooled 5-year data from the Addition trials and a Dutch study reported significant improvements in total cholesterol concentrations in intervention patients compared with control patients (Addition pooled data: -0.27 mmol/L; 95% CI: -0.34 to -0.2 and Dutch trial mean difference -0.2 mmol/L; 95% CI: -0.3 to -0.1). Of the four studies that included MDT as part of their intervention groups, three reported higher reductions in patients BMI compared with control patients.50
The processes of care were evaluated by three studies and all of which reported that those receiving MDT-based care reached their treatment targets defined as HbA1c ≤7% (53 mmol/mol), systolic blood pressure ≤140 mm Hg, total cholesterol ≤4.5 mmol/L, and LDL-cholesterol ≤2.5 mmol/L.50 Process quality measures at 1 year, defined as the proportion of patients receiving guideline-adherent foot examinations, eye examinations, and HbA1c examinations were also higher in the MDT groups compared with the control group. The meta-analysis reported improved patient outcomes in Europe for management approaches that included MDT in addition to other interventions.50
Other systematic global or USA-specific systematic reviews51-55 that assessed an integrated approach to the care of patients with T2DM compared with the usual diabetes care have found improvements in HbA1c, blood pressure, and blood lipid outcomes. Improvements were also reported for increased screening rates for retinopathy, peripheral polyneuropathy, and foot lesions, measuring proteinuria and rate of lipid HbA1c monitoring.53,56,57 Furthermore, one study also reported an economic benefit for integrated care.58 However, two other systematic literature reviews reported only small improvements on patient outcomes or process of care.59,60
Despite the evidence that suggests MDT improves patient outcomes and is cost effective, there is some doubt if the processes used in studies can be effectively replicated in ‘real-world’ situations due to economic pressures on primary care and the large number of patients with T2DM.39,50,56 Furthermore, most studies assessing MDT approaches have limited study periods compared with the time that MDT need to be in place in real-world clinical practice. This hypothesis was tested in a study that assessed the quality of care provided by the Health and Safety Executive Midlands Diabetes, Structured Care Programme that was established in 1997 in Ireland.39 The study found significant improvements in data recording, in the proportion of patients achieving blood pressure and lipid targets over a 16-year period. However, foot assessment and annual review attendance declined in 2016 and only 29% of the patients had all eight of the National Institute for Health and Care Excellence care processes recorded.39
FUTURE OF TYPE 2 DIABETES MELLITUS AND MULTIDISCIPLINARY TEAMS
Physicians and HCP involved in the care of patients with T2DM face several challenges in the future including the management of other comorbidities such as non-alcoholic fatty liver disease (NAFLD), the implementation of new treatment options, and individualised care.
In addition to CVD and renal risk, patients with T2DM have increased susceptibility of NAFLD and higher progression rates to cirrhosis, hepatocellular carcinoma, and death compared with patients with NAFLD without T2DM.61-63 Given the synergistic relationship between NAFLD or non-alcoholic steatohepatitis and T2DM, it is possible to conceive that hepatologists may need to be involved in the MDT care of patients, especially when pharmacological therapies for non-alcoholic steatohepatitis become available.
The evolving treatment landscape, which may, in the future, incorporate precision medicine, using targeted individualised therapy, should be part of the risk and outcome-centred care approach meaning that the place of MDT teams will continue to be pivotal to the success of any future management approaches.








